
Introduction
Previous studies have raised concerns about the possible increased risks of early stent thrombosis and target lesion revascularisation with the Endeavor® Sprint zotarolimus-eluting stent (ZES; Medtronic, Minneapolis, MN, USA) when compared with the CYPHER Select® Plus sirolimus-eluting stent (SES; Cordis, Johnson & Johnson, Warren, NJ, USA)1,2. As these differences appeared to diminish over time, a potential reversal of their safety profiles with prolonged follow-up was suggested1,2. The purpose of this study was to examine 10-year outcomes in patients who received ZES versus SES in the Scandinavian Organization for Randomized Trials with Clinical Outcome (SORT OUT) III trial.
Methods
STUDY DESIGN AND PARTICIPANTS
In brief, SORT OUT III was a randomised, multicentre, open-label, superiority study in which patients aged ≥18 years with stable coronary artery disease or an acute coronary syndrome and at least one target lesion were randomised 1:1 to receive either ZES or SES2. The trial is registered at ClinicalTrials.gov: NCT00660478.
STUDY ENDPOINTS
The pre-specified primary endpoint in SORT OUT III was the composite of cardiac death, myocardial infarction, or target vessel revascularisation. All events occurring up to five years were adjudicated2. This paper reports the composite of death from any cause or myocardial infarction and the individual endpoints of death from any cause, myocardial infarction, and coronary revascularisation, based on a combination of the five-year adjudicated events and registry-based follow-up, using cross-linkage of the Danish National Patient Registry and the Civil Registration System. Myocardial infarctions beyond five years were defined as discharge diagnoses from acute hospital admissions3. Coronary revascularisation was defined as any percutaneous coronary intervention or coronary artery bypass grafting (CABG) from 0-10 years.
STATISTICAL ANALYSIS
We constructed cumulative incidence curves for each endpoint and further estimated crude odds ratios using logistic regression, with patients allocated to SES constituting the reference group.
Results
A total of 2,332 patients with 3,230 lesions were randomly allocated to receive either ZES (1,162 patients, 1,619 lesions) or SES (1,170 patients, 1,611 lesions). As six and 14 had emigrated from the ZES group and SES group, respectively, complete 10-year data for the selected endpoints were available for 2,312 (99%) patients. Ten-year clinical outcomes, including landmark analysis of each event type occurring from years 0 to 5 and from years >5 to 10, respectively, are shown in Table 1. Cumulative incidence curves for each endpoint are shown in Figure 1. None of the outcomes differed significantly between the ZES group and the SES group.
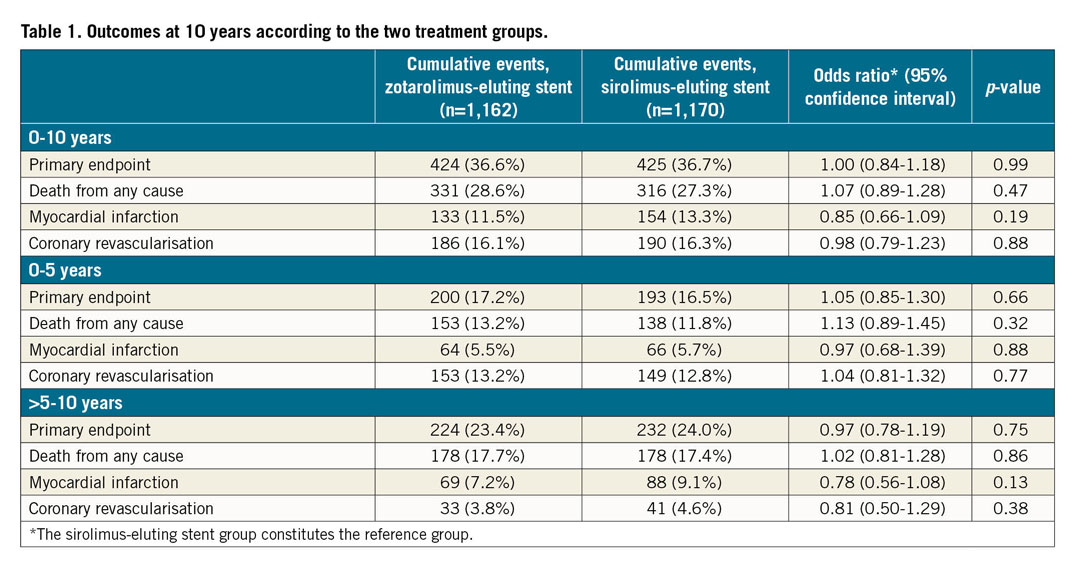
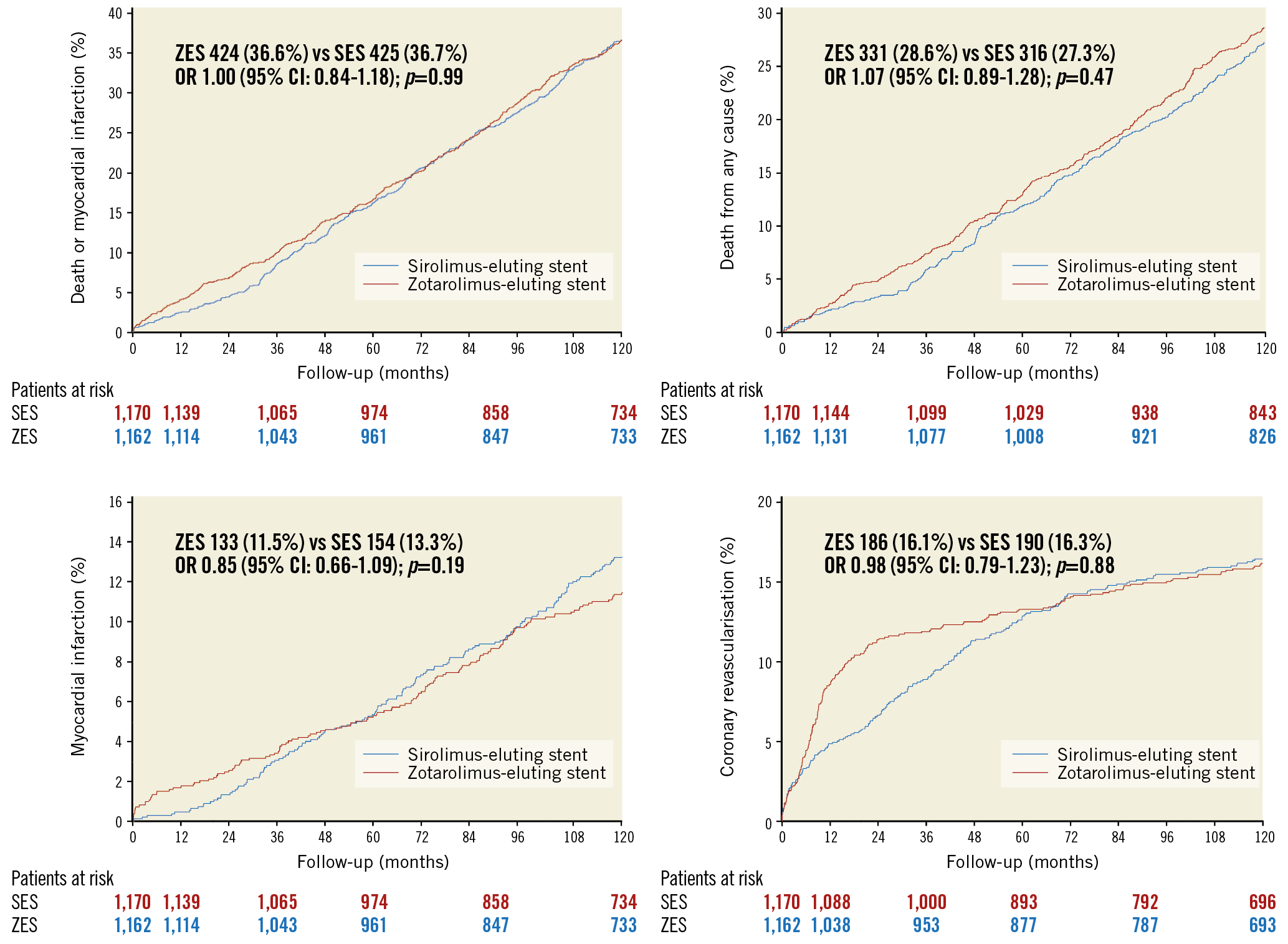
Figure 1. Ten-year event rates in the sirolimus-eluting and zotarolimus-eluting stent groups in SORT OUT III. CI: confidence interval; OR: odds ratio; SES: sirolimus-eluting stent; ZES: zotarolimus-eluting stent
Discussion
The first three commercially available DES comprised the SES, the TAXUS® Express2® paclitaxel-eluting stent (PES; Boston Scientific, Marlborough, MA, USA), and the ZES. Ten-year outcomes from the SORT OUT II and the Sirolimus-Eluting Versus Paclitaxel-Eluting Stents for Coronary Revascularization (SIRTAX) trials comparing SES and PES showed similar risks of major adverse cardiac events, despite the initial results from SIRTAX showing superiority of SES4,5. The Patient Related OuTcomes with Endeavor versus Cypher stenting Trial (PROTECT) compared SES and ZES and found no differences in three-year rates of definite or probable stent thrombosis, but more target vessel revascularisations in the ZES group and more definite stent thrombosis in the SES group1. We found no differences in outcomes at 10 years, corroborating our five-year observations2.
Limitations
Ten-year follow-up in SORT OUT III was not pre-specified, and events beyond five years (0-10 years for the any revascularisation endpoint) were not adjudicated, potentially limiting validity. A related drawback concerned the lack of stent-specific endpoints, including stent thrombosis and target lesion failure and revascularisation, which also hinders proper comparison with prior studies, e.g., PROTECT. Data on dual antiplatelet therapy were not collected, which precluded assessment of the effects of cessation of antiplatelet agents on thrombotic events. Finally, the evaluated stents are no longer commercially available.
Conclusion
Our study showed comparable 10-year rates of death from any cause, myocardial infarction, and coronary revascularisation with ZES and SES in patients with acute or stable coronary artery disease. It is possible that, with increasing follow-up, progressive atherosclerosis and competing conditions may play an increasingly important prognostic role.
|
Impact on daily practice In a randomised controlled trial, we found comparable 10-year outcomes with zotarolimus-eluting stents and sirolimus-eluting stents in patients with acute or stable coronary artery disease. Therefore, it is possible that, with increasing follow-up, progressive atherosclerosis and competing conditions may play a more important prognostic role compared with the index lesion and the drug-eluting stent itself. In other words, aggressive secondary prevention of atherosclerosis appears to be particularly important in order potentially to reduce ischaemic events after stent implantation. |
Acknowledgements
We thank Helle Bargsteen for her efficient help with all aspects of the trial.
Funding
SORT OUT III was supported by unrestricted research grants from Cordis and Medtronic.
Conflict of interest statement
M. Pareek has the following relationships – advisory board and speaking honoraria from AstraZeneca, speaking honoraria from Bayer and Boehringer Ingelheim. S.D. Kristensen has the following relationships – lecture fees from AstraZeneca, Bayer, and BMS/Pfizer. M. Maeng has the following relationships – advisory board and speaking honoraria from AstraZeneca, Bristol-Myers Squibb, Novo Nordisk, Boehringer Ingelheim, and Bayer, research grants from Volcano (now Philips). The other authors have no conflicts of interest to declare.

