Abstract
Aims: Sirolimus-eluting stents (SES) have been shown to be superior to Endeavor zotarolimus-eluting stents (ZES) and comparable to Resolute ZES at eight-month angiography in patients treated for total coronary occlusions (TCO). This study investigated clinical outcome at three-year follow-up.
Methods and results: The PRISON III trial investigated the efficacy and safety of SES against ZES (Endeavor and Resolute) in two study phases. In the first phase, 51 patients were randomised to receive SES and 46 to Endeavor ZES. In the second phase, 103 and 104 patients were randomised to SES or Resolute ZES, respectively. Between one and three years there were only a few additional clinical events in all groups. As a result, the rates of target lesion revascularisation 12.2% vs. 19.6%, p=0.49, target vessel failure 14.3% vs. 19.6%, p=0.68, and definite or probable stent thrombosis 4.1% vs. 2.2% were comparable between SES and Endeavor ZES at three years. In the second study phase, the rates of target lesion revascularisation 10% vs. 5.9%, p=0.42, target vessel failure 10% vs. 7.9%, p=0.79 and definite or probable stent thrombosis 1.0% vs. 0% were similar between SES and Resolute ZES.
Conclusions: The present study demonstrated a low incidence of clinical events between one- and three-year follow-up with either SES compared to Endeavor ZES or SES versus Resolute ZES in patients treated for total coronary occlusions.
Introduction
In the PRISON III trial, we investigated the Endeavor® (Medtronic, Minneapolis, MN, USA) and its successor the Resolute® zotarolimus-eluting stent (also Medtronic) (Endeavor ZES, Resolute ZES) against the first-generation sirolimus-eluting stent (SES) (CYPHER®; Cordis, Johnson & Johnson, Warren, NJ, USA) in patients with total coronary occlusions/chronic total occlusions (TCO/CTO)1. At eight-month angiographic follow-up, SES was superior to Endeavor ZES and comparable to Resolute ZES with respect to late lumen loss. In this study, we investigated whether the change in late lumen loss among these three stent platforms translated into an important difference in clinical outcome at three years.
Methods
The methods and primary angiographic and clinical endpoints at 12 months of the PRISON III study have been published previously1,2. In summary, any death, target-related myocardial infarction (MI) or ischaemia-driven target lesion revascularisation (TLR) was recorded as a major adverse cardiac event (MACE). Other clinical endpoints were ischaemia-driven target vessel revascularisation (TVR), target vessel failure (TVF; the composite of cardiac death, target-related MI and TLR), the incidence of probable/definite stent thrombosis (ST) and the combined endpoint of TVF and asymptomatic reocclusion, defined as target lesion reocclusion at eight-month reangiography without the performance of any revascularisation. Finally, a total count was registered of all clinical events as well as repeated events including any death, any MI and any revascularisation.
Results
Data on study enrolment and patient characteristics have been published previously1. Clinical follow-up was obtained in 98% of patients at three years. Between one- and two-year follow-up, only a few additional clinical events occurred in both the SES versus Endeavor ZES and SES versus Resolute ZES comparisons. In the first phase, the SES group experienced one additional cardiac death, which occurred due to low output failure, and one patient experienced a myocardial infarction due to a very late ST following target lesion revascularisation compared to two ischaemia-driven percutaneous revascularisations in the Endeavor ZES group. In the second phase, the Resolute ZES group demonstrated two non-cardiac deaths due to malignancy and two against four ischaemia-driven percutaneous revascularisations compared to the SES group, with no registered stent thrombosis for both groups. As a result, there were no significant differences between both the SES versus Endeavor ZES and SES versus Resolute ZES with regard to MACE, TVF (Figure 1A, Figure 1B), definite or probable ST and the combined rate of TVF and asymptomatic reocclusion at three-year follow-up (Table 1). The clinical outcome in the subgroup of CTOs is demonstrated in the lower panel of Table 1.
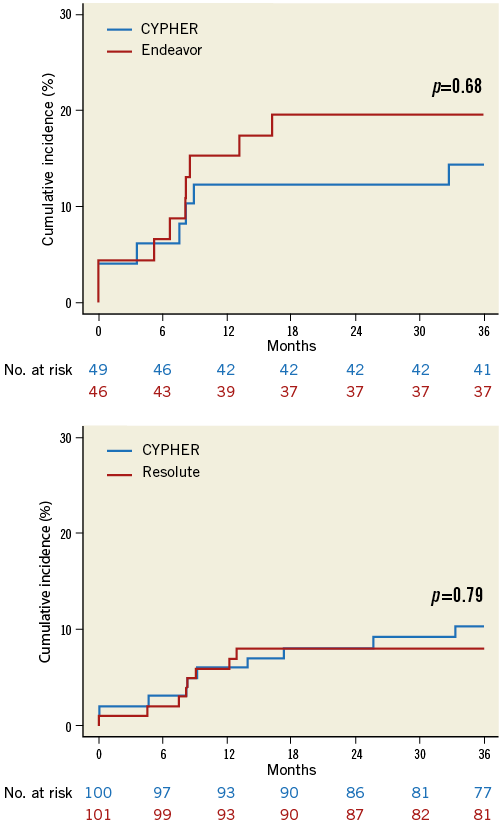
Figure 1. Cumulative incidence of target vessel failure. A) Comparison of SES (CYPHER) with Endeavor ZES. B) Comparison of SES (CYPHER) with Resolute ZES.
Discussion
In this study, the occurrence of clinical events between one- and three-year follow-up was low with either SES compared to Endeavor ZES or SES versus Resolute ZES in patients treated for TCO/CTO. This study was not powered for clinical endpoints and results therefore remain exploratory.
The relative short duration of neointima inhibition with Endeavor ZES resulted in a higher rate of late lumen loss against SES at eight months1. At three years, this did not translate into a significant increment of clinical events in patients with TCO/CTO. On the contrary, other large “all-comer” trials (SORT OUT III and PROTECT) observed a significant increase in target vessel revascularisations at three years (SORT OUT III, 9.1% vs. 6.4%, hazard ratio [HR] 1.40, p=0.02; PROTECT 8.1% vs. 7.1%, HR 1.19, p=0.03) with comparable rates of myocardial infarction and cardiac death comparing Endeavor ZES against SES3,4. Despite these dissimilarities, our results demonstrated a considerably higher absolute rate of target vessel revascularisations, which emphasises the importance of selective randomised trials for this complex lesions subset of TCO/CTO.
We found a low rate of ST in both groups in the first phase. One late definite ST was observed in the Endeavor ZES group as opposed to a subacute probable ST and one angiographically determined very late ST in the SES group between two and three years. Interestingly, these observations reflect the results of the all-comer PROTECT trial, powered for stent thrombosis. They noted an increased risk for probable and definite late ST with the Endeavor ZES, whereas SES showed an increased risk for very late definite ST4. The differential mechanism remains speculative; however, the late ST with the Endeavor ZES could be attributed to a higher risk of thrombosis due to restenosis. On the other hand hypersensitivity reactions to SES polymers, leading to uncovered and malapposed stent struts, might explain the higher incidence of very late ST5.
In the second phase, we showed no cardiac deaths and comparable rates of myocardial infarction, TVR, TVF and MACE between SES and Resolute ZES, with low rates of definite or probable ST at three years. Our results support earlier findings from the LONG DES IV trial, investigating SES and Resolute ZES in a subset of long lesions (>25 mm) with comparable rates of TVF 14.4% vs. 16.0%, p=0.62, at 12 months with low incidence of ST6. Other all-comer trials investigating the Resolute ZES against everolimus-eluting stents (the RESOLUTE All Comers and TWENTE trials) also found equal rates of target vessel failure and low rates of ST at two years7,8. Consequently, we consider both SES and Resolute ZES safe and effective in patients with TCO/CTO. However, it should be noted that the PRISON II trial demonstrated an increased risk of definite ST with SES in TCO at five years9. Hence, long-term clinical follow-up beyond three years remains imperative to assess the risk of very late ST between SES and both the Endeavor and Resolute ZES in this group of patients with TCO/CTO.
Conclusion
The present study demonstrated a low incidence of clinical events between one- and three-year follow-up with either SES compared to Endeavor ZES or SES versus Resolute ZES in patients treated for total coronary occlusions.
| Impact on daily practice In this study we investigated the three-year clinical outcomes comparing first-generation sirolimus-eluting stents (SES, CYPHER) against second-generation zotarolimus-eluting stents (Endeavor and Resolute ZES) in TCO/CTO. We consider both SES and Resolute ZES safe and effective in these complex lesions. Due to commercial availability, we recommend using the Resolute ZES in daily clinical practice for treating TCO/CTO. |
Funding
This study was supported by a research grant from the Cordis Corporation.
Conflict of interest statement
J.J. Koolen has received research grants from Biotronik. J.P. Henriques has received research grants from Abbott Vascular and Abiomed. The other authors have no conflicts of interest to declare.

