
Abstract
Aims: The aim of this report was to assess the three-year safety and efficacy of implanting newer-generation Resolute Integrity zotarolimus-eluting stents (ZES) versus PROMUS Element everolimus-eluting stents (EES) in all-comers.
Methods and results: In the randomised, multicentre, investigator-initiated DUTCH PEERS trial, a total of 1,811 all-comers were 1:1 randomly assigned to treatment with ZES versus EES. A total of 1,293 patients (72%) were treated for complex lesions and 455 patients (25%) were treated for multiple lesions. The primary endpoint target vessel failure (TVF) is a composite of cardiac death, target vessel-related myocardial infarction or target vessel revascularisation. Adverse clinical events were independently adjudicated. Three-year follow-up data were obtained in 1,807 patients (99.8%, four withdrawals). Both the ZES and EES groups showed favourable outcomes with a similar incidence of TVF (10.7% vs. 10.3%; pLog-rank=0.77) and the individual components thereof: cardiac death (3.2% vs. 3.1%; pLog-rank=0.87), target vessel-related myocardial infarction (2.8% vs. 2.2%; pLog-rank=0.44) and target vessel revascularisation (6.0% vs. 6.2%; pLog-rank=0.87). In addition, the incidence of definite or probable stent thrombosis was similar for patients treated with ZES versus EES (1.4% vs. 1.1%; pLog-rank=0.66).
Conclusions: The safety and efficacy of treating all-comers with newer-generation Resolute Integrity and PROMUS Element stents was found to be extended up to three years.
Abbreviations
DES: drug-eluting stent
EES: everolimus-eluting stent
MI: myocardial infarction
TVF: target vessel failure
ZES: zotarolimus-eluting stent
Introduction
Newer-generation metallic drug-eluting stents (DES), such as the cobalt-chromium-based Resolute Integrity® zotarolimus-eluting stent (ZES) (Medtronic, Minneapolis, MN, USA) and the platinum-chromium-based PROMUS Element™ everolimus-eluting stent (EES) (Boston Scientific, Marlborough, MA, USA), have stent designs that were developed to facilitate deliverability and further improve DES apposition1,2. Both DES were compared for the first time in the randomised DUTCH PEERS trial, which was also the first trial ever to investigate Resolute Integrity. In 1,811 all-comers, this study demonstrated non-inferiority of the ZES versus the EES at 12-month follow-up1. Long-term data from the DUTCH PEERS trial are of interest, as certain between-stent differences may only be discovered after years. We therefore assessed the three-year clinical outcome of percutaneous coronary interventions in the two DES arms.
Methods
The design of the study, definitions of clinical endpoints and characteristics of patients, lesions and procedures of the multicentre, patient-blinded, investigator-initiated, 1:1 randomised DUTCH PEERS (TWENTE II) trial (ClinicalTrials.gov NCT01331707) have been reported previously1. DUTCH PEERS enrolled 1,811 patients with any type of clinical syndrome, including 20.4% of patients with ST-elevation myocardial infarction (MI), 24.7% with non-ST-elevation MI and 71.7% with complex coronary lesions1. The external CRO Diagram (Zwolle, the Netherlands) monitored clinical outcome in 10% of randomly selected patients and organised the adjudication of adverse events by an independent clinical events committee for both two- and three-year follow-up. The DUTCH PEERS trial complied with the Declaration of Helsinki and was approved by the Medical Ethics Committee Twente and the institutional review boards of all participating centres. All patients provided written informed consent. Clinical endpoints were defined according to the Academic Research Consortium (ARC), including the addendum on MI3,4. The primary endpoint was target vessel failure (TVF) at one year, a composite of cardiac death, target vessel-related MI and clinically indicated target vessel revascularisation. P-values and confidence intervals were two-sided and p-values <0.05 were considered significant. Statistics were performed as previously reported4, using SPSS, Version 22.0 (IBM Corp., Armonk, NY, USA).
Results
Three-year follow-up was obtained from 1,807 patients (99.8%; four consent withdrawals). Patients treated with Resolute Integrity ZES (n=906) and PROMUS Element EES (n=905) showed favourable outcomes with similar TVF rates (10.7% vs. 10.3%; pLog-rank=0.77) (Table 1, Figure 1). The incidence of the individual components of TVF was similar for both DES: cardiac death (3.2% vs. 3.1%; pLog-rank=0.87); target vessel-related MI (2.8% vs. 2.2%; pLog-rank=0.44); and target vessel revascularisation (6.0% vs. 6.2%; pLog-rank=0.87) (Table 1, Figure 1). At three-year follow-up, 6.1% of patients treated with Resolute Integrity ZES and 7.1% of patients treated with PROMUS Element ZES were on dual antiplatelet therapy.
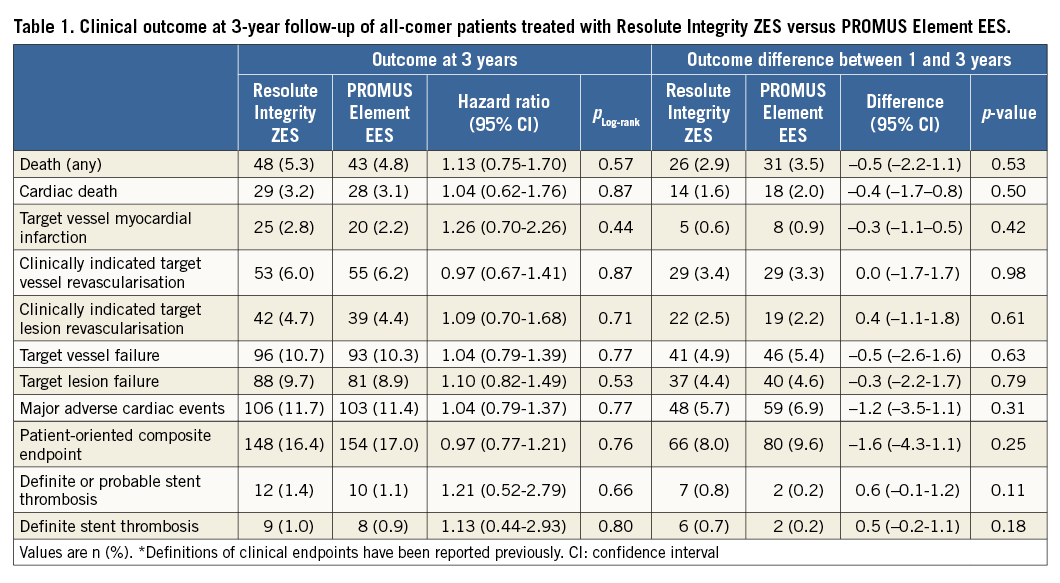
Figure 1. Kaplan-Meier curves for target vessel failure and individual components. A) Target vessel failure. B) Cardiac death. C) Target vessel-related myocardial infarction. D) Target vessel revascularisation. Patients treated with Resolute Integrity (red) versus PROMUS Element stents (grey).
As shown in Table 1 and Figure 2, the rates of definite and definite or probable stent thrombosis were low for patients treated with Resolute Integrity ZES and PROMUS Element EES (1.0% vs. 0.9%; pLog-rank=0.80 and 1.4% vs. 1.1%; pLog-rank=0.66, respectively). Due to an apparent dissimilarity between the Resolute Integrity ZES and PROMUS Element EES groups in the course of their time-to-event curves for definite or probable stent thrombosis (catch-up after >1 year vs. main increase within first 12 months) (Figure 2), we performed post hoc a landmark analysis at 12-month follow-up. Definite or probable stent thrombosis occurred during the first year in 0.6% vs. 0.9% (pLog-rank=0.41) of patients and during the second plus third years in 0.8% vs. 0.2% (pLog-rank=0.09) of patients.
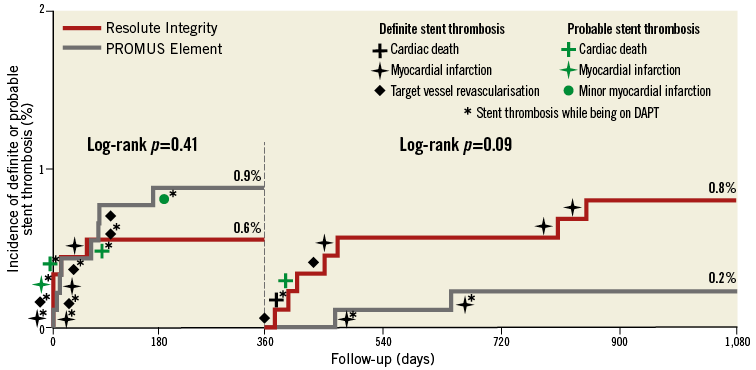
Figure 2. Kaplan-Meier curve for stent thrombosis. Patients treated with Resolute Integrity (red) versus PROMUS Element stents (grey). Dual antiplatelet therapy (DAPT) means acetylsalicylic acid plus P2Y12 receptor antagonist.
Discussion
The current study reports the three-year clinical outcome of the DUTCH PEERS trial, which is the first randomised study to compare Resolute Integrity ZES versus PROMUS Element EES in all-comers1. Patients of both stent arms had similar and relatively low rates of the main clinical endpoint TVF (10.7% vs. 10.3%), and the incidence of definite or probable stent thrombosis was low and comparable (1.4% vs. 1.1%).
Our findings corroborate the results of previous randomised studies, that compared the predecessors of these newer-generation DES in broad patient populations5,6. Long-term outcome data about Resolute Integrity ZES and PROMUS Element EES in all-comers are scarce. The only randomised trial other than DUTCH PEERS that studied Resolute Integrity in all-comers is the SORT OUT VI study2, but definite long-term results of that study have not yet been published. In addition, the PLATINUM trial is the only randomised trial that has published three-year follow-up data on the use of PROMUS Element in low-to-moderate risk patients (e.g., ≤2 de novo lesions in vessels ≥2.5 mm), showing similar safety and efficacy for both PROMUS Element and XIENCE V® (Abbott Vascular, Santa Clara, CA, USA)7. Our current three-year follow-up data support these favourable findings in a broader, greatly unrestricted patient population.
As the present study was not powered to assess between-group differences in secondary clinical endpoints, these findings should be considered hypothesis-generating.
Conclusions
The safety and efficacy of treating all-comers with newer-generation Resolute Integrity and PROMUS Element stents in the randomised DUTCH PEERS trial was extended up to three years.
| Impact on daily practice The three-year results of the DUTCH PEERS trial are the first long-term data in all-comers from a randomised comparison of the newer-generation Resolute Integrity ZES and the PROMUS Element EES, two stents that are often used in routine clinical practice. The consistently low rates of adverse clinical events, such as target vessel myocardial infarction, target vessel revascularisation and definite or probable stent thrombosis, provide a strong signal of sustained safety and efficacy of both metallic drug-eluting stents in this broad patient population. These long-term outcome data fill a gap in the literature and might in the future be useful for interpreting long-term data following broader applications of bioresorbable scaffolds. |
Funding
This investigator-initiated study was supported equally by Boston Scientific and Medtronic.
Conflict of interest statement
C. von Birgelen was a consultant to Boston Scientific and Medtronic, and has received lecture fees from AstraZeneca and Biotronik. The institution has received research grants provided by AstraZeneca, Biotronik, Boston Scientific, and Medtronic. The other authors have no conflicts of interest to declare.

