
Abstract
Aims: The COMBO stent combines sirolimus elution with an endothelial progenitor cell-capturing layer to promote early endothelialisation. There has not been a head-to-head comparison of this novel device with any other currently used drug-eluting stent (DES). We sought to compare clinical outcome at two years after COMBO stent placement with the Resolute Integrity or PROMUS Element stent in an all-comers cohort.
Methods and results: Patients from the REMEDEE registry (COMBO, n=1,000) were matched with patients from the DUTCH PEERS trial (PROMUS Element/Resolute Integrity, n=1,811). Propensity score matching on 13 baseline characteristics was applied to create two balanced cohorts of patients treated with COMBO versus PROMUS Element/Resolute Integrity. Propensity score matching yielded 771 patient pairs, representing all-comers patients, with a median age of 65 years, 27% female and more than 50% of patients presenting with acute coronary syndrome. Target lesion failure (TLF), a composite of cardiac death, target vessel MI and any target lesion revascularisation, at two-year follow-up was 7.9% in COMBO and 6.4% in PROMUS Element/Resolute Integrity, HR 1.24 (95% CI: 0.85-1.81), p=0.26. Definite stent thrombosis (ST) was not significantly different between groups (0.8% vs. 0.9%, p=0.79).
Conclusions: In a propensity-matched analysis, the COMBO stent showed similar rates of TLF and ST at two-year follow-up compared to Resolute Integrity and PROMUS Element.
Abbreviations
CABG: coronary artery bypass grafting
DES: drug-eluting stent
EPC: endothelial progenitor cell
MI: myocardial infarction
NSTE-ACS: non-ST-segment elevation acute coronary syndrome
PCI: percutaneous coronary intervention
ST: stent thrombosis
STEMI: ST-segment elevation myocardial infarction
TLF: target lesion failure
TLR: target lesion revascularisation
Introduction
Clinical outcomes after percutaneous coronary intervention (PCI) with stent placement are strongly related to the device type that is implanted. Drug-eluting stents (DES) improved outcomes compared to bare metal stents in terms of in-stent restenosis1-3. Further developments in coronary DES led to different metal platforms, drug types, mechanisms of drug release, and polymer coatings4. A novel stent that has been developed to reduce adverse outcomes after PCI further is the dual-therapy COMBO™ stent (OrbusNeich Medical BV, Hoevelaken, the Netherlands)5,6.
The COMBO stent combines a biodegradable abluminal sirolimus-eluting polymer with a unique luminal endothelial progenitor cell-capturing layer. This stent technique aims to promote true vessel healing and thereby improve clinical outcome after PCI. Two-year clinical outcomes after COMBO stent deployment were evaluated in the all-comers REMEDEE registry7,8. Thus far, no randomised data are available comparing the dual-therapy stent technology with new-generation DES such as the Resolute Integrity® zotarolimus-eluting stent (Medtronic, Minneapolis, MN, USA) and the PROMUS Element™ everolimus-eluting stent (Boston Scientific, Marlborough, MA, USA)9-11. These newer-generation durable polymer drug-eluting stents have been shown to be similarly safe and efficacious11.
The objective of the current analysis was to compare the two-year clinical outcomes of the COMBO stent with Resolute Integrity and PROMUS Element stent therapy in a balanced cohort. This analysis is the first to compare results after COMBO stent placement with other DES and will provide additional insight into the clinical performance of the COMBO stent.
Methods
DEVICES
The three investigational devices are the PROMUS Element, the Resolute Integrity and the COMBO stent. All stents are CE-marked (available on the European market) and used in daily clinical practice. The PROMUS Element consists of a thin fluoropolymer everolimus coating on a stent platform made from a platinum-chromium alloy which has a novel, laser-cut, open-cell stent design, consisting of serpentine rings connected by helically distributed links9. The Resolute Integrity consists of a BioLinx zotarolimus coating on a cobalt-chromium alloy stent platform that has a sinusoidal design. The BioLinx polymer system of this stent consists of a blend of three different polymers: the hydrophobic C10 polymer, aiding drug release; a hydrophilic C19 polymer, supporting biocompatibility; and a polyvinylpyrrolidone, which increases the initial drug burst and enhances the elution rate10. The COMBO consists of a 316L stainless steel alloy in a helical sinusoidal design and an abluminal biodegradable sirolimus-eluting polymer layer, with a bio-engineered anti-CD34+ antibody layer to attract endothelial progenitor cells.
TRIALS
The DUTCH PEERS trial is a prospective, investigator-initiated, all-comers, randomised (1:1), non-inferiority trial comparing clinical outcomes after PROMUS Element and Resolute Integrity stent placement. The study design and primary results have been published in more detail elsewhere11. In short, a total of 1,811 patients were enrolled between November 2010 and May 2012 at four PCI centres in the Netherlands (PROMUS Element, N=905, Resolute Integrity, N=906). Based on the primary endpoint of target vessel failure, non-inferiority of the Resolute Integrity stent was proven, compared with the PROMUS Element stent, and both stents were similarly efficacious and safe in an all-comers population11. At two-year follow-up, similar clinical outcomes were reported for both DES examined12.
The REMEDEE registry is a prospective, investigator-initiated, all-comers, single-arm registry of 1,000 patients treated with the COMBO stent. The study was conducted in nine European sites across the Netherlands, Latvia, Luxembourg, Northern Ireland and Spain. Enrolment started in June 2013 and was completed in March 2014. Registry design and clinical results have been published previously7,8. All events were adjudicated by an independent clinical events committee.
In the REMEDEE registry, DAPT regimen was advised to follow current guidelines: six to 12 months after elective PCI, 12 months after acute coronary syndrome (ACS). The DUTCH PEERS trial recommended prescription of aspirin and clopidogrel for 12 months after PCI. Both studies complied with the Declaration of Helsinki, and were approved by the independent medical ethics committees and institutional review boards of all participating centres. The DUTCH PEERS trial complied with the CONSORT 2010 statement. All patients in the studies provided written informed consent.
PATIENT POPULATION
Inclusion criteria were identical between the two studies. The following inclusion criteria were used: patients undergoing PCI with stent treatment, >18 years old and willing and able to cooperate with study requirements. Exclusion criteria in both trials were: high probability of non-adherence to the follow-up requirements (due to social, psychological or medical reasons), currently participating in another investigational drug or device study in which a routine angiographic follow-up is planned, a life expectancy of <1 year. Additional exclusion criteria for the DUTCH PEERS trial were: known pregnancy or intolerance to a P2Y12 receptor antagonist, aspirin, heparin or any of the components of the DES.
CLINICAL ENDPOINTS AND DEFINITIONS
The primary outcome of interest was target lesion failure (TLF), a composite endpoint of cardiac death, target vessel-related myocardial infarction (MI), or any target lesion revascularisation at two years. Stent thrombosis (definite, definite or probable) at two years was evaluated. Two-year follow-up was defined as 720 days post index procedure. Endpoints were defined according to the Academic Research Consortium including the addendum on myocardial infarction13,14. Furthermore, we looked at the individual components of the endpoint TLF separately, i.e, cardiac death, target vessel MI and target lesion revascularisation.
STATISTICAL ANALYSIS (ENTIRE COHORT)
Categorical variables are presented as numbers and percentages and are compared for the entire cohort with Fisher’s exact test. Based on their distributions, continuous variables are presented as mean±standard deviation or median with interquartile ranges and compared with the Student’s t-test or Wilcoxon rank-sum test, or Kruskal-Wallis test in case of non-normally distributed variables.
PROPENSITY SCORE MATCHING
A pre-specified analysis plan was made prior to the conduct of the analysis to eliminate (potential) model bias, including a consensus on the matching variables. Propensity score matching was performed using the following thirteen selected baseline variables: age, gender, insulin-treated diabetes mellitus, hypertension, previous MI, previous PCI, previous bypass, ACS, number of treated lesions, target vessel location, stent length and diameter, and American College of Cardiology/American Heart Association (ACC/AHA) classification. A logistic multivariable regression was used with device type (PROMUS Element or Resolute Integrity versus COMBO) as dependent variable and the 13 above listed baseline variables as independent predictors to calculate the propensity score. Patients were one-to-one greedy matched using the nearest neighbour method, COMBO versus either PROMUS Element or Resolute Integrity. The calliper for the propensity match was set at 0.2.
All statistical analyses were conducted in R Studio and R version 3.2.2 and the package MatchIt for propensity matching 17 and 18 (The R Foundation for Statistical Computing, Vienna, Austria). All reported p-values were two-tailed, and p<0.05 was considered statistically significant.
SENSITIVITY ANALYSES
Sensitivity analyses were performed per stent device (COMBO versus Resolute Integrity, COMBO versus PROMUS) using the same method of propensity score matching.
ANALYSIS OF THE MATCHED COHORT
Baseline variables of the matched cohort were compared with the same methods as the unmatched cohorts. Kaplan-Meier estimates were used for the cumulative incidence of outcomes at two-year follow-up. P-values and hazard ratios were calculated using Cox proportional hazards (CPH) models. CPH assumptions were visually inspected by plotting Schoenfeld residuals. P-values <0.05 were considered statistically significant.
Results
PATIENT AND ANGIOGRAPHIC CHARACTERISTICS FROM THE DUTCH PEERS TRIAL AND THE REMEDEE REGISTRY
The baseline characteristics of both trials have been published previously7,9, and are summarised in Supplementary Table 1. REMEDEE registry patients are older, have more hypertension, more previous PCI and previous MI. Acute coronary syndrome was more present in the DUTCH PEERS trial, and the RCA was treated more frequently in DUTCH PEERS. Significant differences were also found in the number of treated lesions, AHA/ACC lesion type and lesion length.
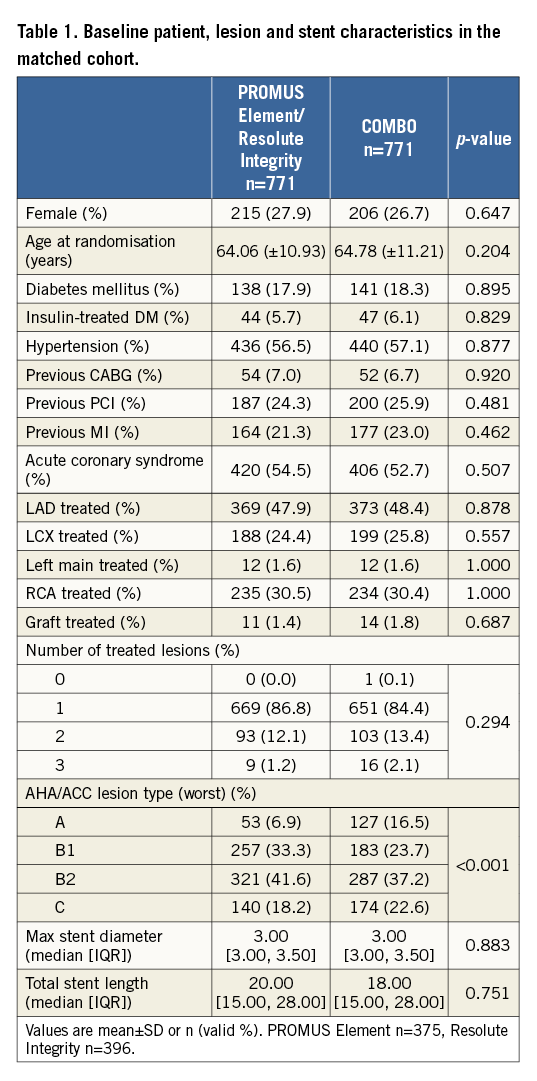
PATIENT AND ANGIOGRAPHIC CHARACTERISTICS OF THOSE IN THE MATCHED COHORTS
The propensity score calculation was performed taking into account the thirteen baseline and angiographic variables. The propensity score match resulted in 771 balanced patient pairs, as illustrated in Table 1. AHA/ACC lesion type could not be perfectly matched (taking into account the four lesion types). The combined type B2/C was the same, both 59.8%.
CLINICAL OUTCOMES AT TWO YEARS IN THE MATCHED COHORTS
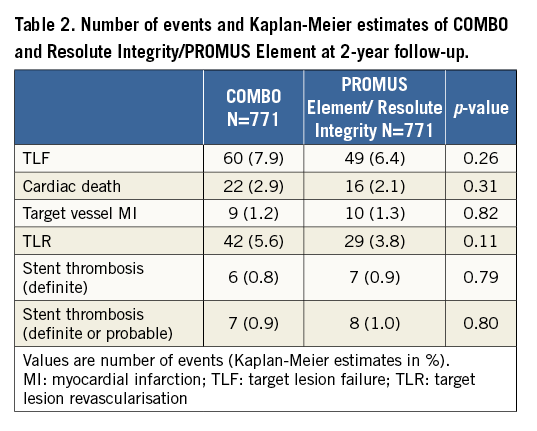
TLF at two-year follow-up in the matched cohort by Kaplan-Meier estimate is shown in Figure 1. TLF occurred in 7.9% of patients treated with the COMBO, and in 6.4% of patients treated with PROMUS Element/Resolute Integrity (p=0.26), HR 1.24 (95% CI: 0.85-1.81). Cardiac death (Figure 2A) occurred in 2.9% versus 2.1% (p=0.31), HR 1.39 (95% CI: 0.73-2.65); target vessel MI (Figure 2B) in 1.2% versus 1.3% (p=0.82), HR 0.90 (95% CI: 0.37-2.22); and TLR (Figure 2C) in 5.6% versus 3.8% (p=0.11), HR 1.48 (95% CI: 0.92-2.37), respectively. Definite stent thrombosis occurred in 0.8% of the COMBO patients and in 0.9% of the PROMUS Element/Resolute Integrity patients (p=0.79), HR 0.86 (95% CI: 0.29-2.57) (Figure 3). Definite or probable stent thrombosis was observed in 0.9% versus 1.0% of patients (p=0.8), HR 0.88 (95% CI: 0.32-2.43), respectively. Results are presented in Table 2.
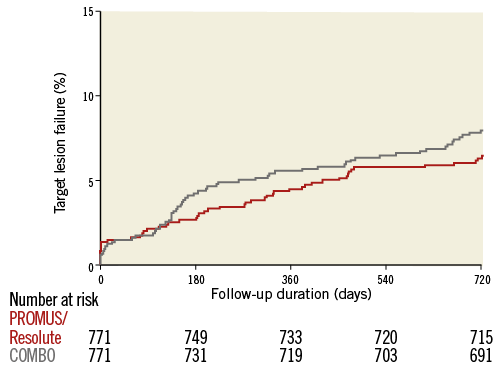
Figure 1. Target lesion failure by Kaplan-Meier method.
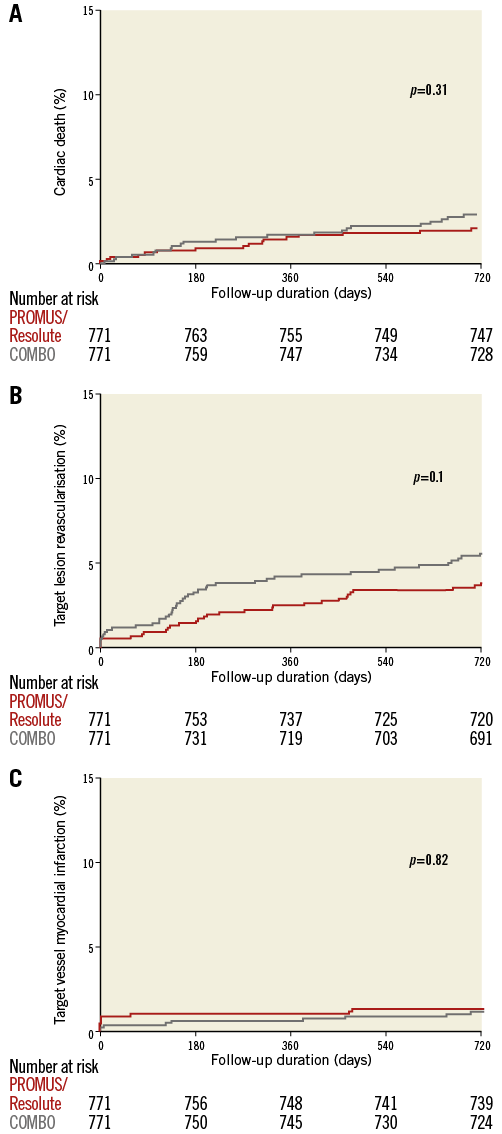
Figure 2. Cardiac death, TLR and TV-MI by Kaplan-Meier method.
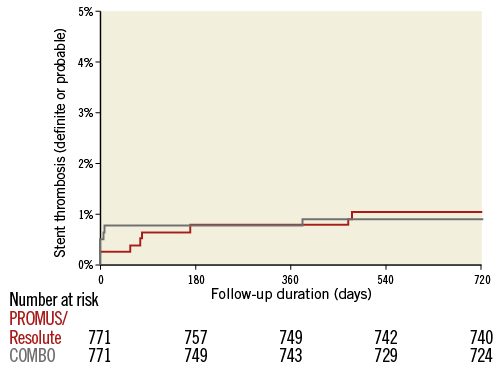
Figure 3. Definite or probable stent thrombosis by Kaplan-Meier method.
SENSITIVITY ANALYSES
Sensitivity analyses were conducted per device type. Two sets of patient pairs were made for COMBO and Resolute Integrity (both n=588). Baseline and angiographic characteristics were nicely balanced (Supplementary Table 2). The evaluation of TLF at two-year follow-up shows 8.4% for COMBO and 7.2% for Resolute Integrity (p=0.43) (Supplementary Table 3). Sensitivity analysis for the comparison of COMBO with PROMUS Element also resulted in two balanced cohorts (both n=617), and evaluation of TLF showed 7.4% for COMBO and 5.5% for PROMUS Element (p=0.19) (Supplementary Table 4, Supplementary Table 5).
Discussion
This is the first analysis comparing the bio-engineered COMBO stent with newer-generation DES, showing no significant differences in clinical outcomes between the COMBO and PROMUS/Resolute Integrity in a balanced cohort at two-year follow-up. Furthermore, the current analysis shows that baseline clinical and angiographic characteristics are different among all-comers trials, and low rates of target lesion failure are observed at two-year follow-up after DES implantation.
COMBO VERSUS CURRENT MONOTHERAPY DES
This analysis is the first to compare the COMBO stent with current DES. No significant differences were observed in terms of TLF, any individual endpoints and definite/probable stent thrombosis. Target lesion revascularisation was non-significantly higher in patients treated with COMBO, with an increase of revascularisation between six and nine months post index procedure. Although there was no scheduled repeat angiography in the REMEDEE registry, there might be a slight recall bias due to the fact that the COMBO stent is a newer device, and patients with persistent angina were more easily scheduled for repeat angiography.
ALL-COMERS PCI COHORTS
In the present analysis we found that the baseline characteristics of two large all-comers PCI patient cohorts were different, despite similar inclusion and exclusion criteria. When comparing clinical and angiographic characteristics, there were notable differences in age, hypertension, previous PCI or MI, location of lesion, number of lesions, lesion type and lesion length. This could be explained by the fact that the DUTCH PEERS trial is a Dutch trial, where patients were enrolled in the Netherlands only, while the REMEDEE registry enrolled patients in five European countries. Also, there might be differences in patients who want to participate in a randomised trial or registry. De Boer et al previously compared participants and non-participants of a single high-volume centre in two “all-comer” randomised PCI trials and found that these two groups differed significantly in baseline characteristics and clinical outcome; the authors explained this by the fact that only half of the target population was enrolled15. While the all-comers design of clinical studies may not always fully represent “real-world” clinical practice, von Birgelen et al recently found that five-year clinical outcome was similar for participants in a randomised “most-comer” DES study with a very high enrolment rate versus the complete cohort of patients who had been eligible for trial enrolment16.
If we compare the baseline characteristics with other trials (LEADERS17, COMPARE18, SPIRIT IV19), baseline characteristics are similar but do alter frequently, e.g., age (lowest mean: 63 years, highest: 65 years), hypertension (lowest: 44%, highest: 77%), previous PCI (lowest: 13%, highest: 37%), previous MI (lowest: 15%, highest: 33%). Attention should be given to baseline characteristics when interpreting all-comers PCI trial results. In this study we accounted for multiple confounders by propensity score matching; there were no differences between COMBO and PROMUS Element/Resolute Integrity patients.
CLINICAL OUTCOMES TWO YEARS AFTER STENT PLACEMENT
In the PRODIGY study, subgroup analyses were performed comparing the clinical combined endpoint of death, MI and target vessel revascularisation after BMS or DES placement at two years. The endpoint occurred in 32.1% of patients treated with BMS20. In this same study, the endpoint occurred in 27.8% of patients treated with zotarolimus-eluting Endeavor® Sprint stents (ZES-S) (Medtronic), 26.2% in paclitaxel-eluting stents (PES) and 19.2% in everolimus-eluting stents (EES). A major decrease in adverse events was noted with the use of newer DES.
The adverse event rates after PCI continue to decrease. In the LEADERS trial two-year results, a further decline in event rates was noticed, comparing the first-generation DES, a sirolimus-eluting stent, with second-generation DES, a biolimus-eluting stent. The combined cardiac death, MI and clinically indicated TLR rate was 11.9% in BES and 13.6% in SES21. The COMPARE trial two-year results also showed lower event rates with second-generation DES (rate of all death, MI, and TVR: 9.0% in EES and 13.7% in PES)22. SPIRIT IV two-year results were also in line with these findings, showing lower events in second-generation DES23. To evaluate the efficacy of the bio-engineered anti-CD34 antibody layer, a comparison might be carried out with a DES eluting sirolimus. If we compare the two-year results of this balanced cohort with monotherapy SES from LEADERS, we notice a lower event rate in the COMBO stent group.
The theoretical benefit of the novel dual-therapy stent technology is to reduce the healing time and potentially reduce the DAPT duration. This could specifically benefit patients who have a high risk of bleeding or are scheduled for operation, or patients who are unlikely to adhere to medication. The BioFreedom™ stent (Biosensors Interventional Technologies, Singapore) with one month DAPT has been demonstrated to be safer than BMS in patients with a high risk of bleeding23. Myocardial infarction and/or stent thrombosis was observed in 8.2% of patients with a high risk of bleeding treated with BioFreedom at two-year follow-up. These data cannot be directly compared to our data, due to patient selection. Future trials would be needed to address the differences in performance between BioFreedom and the COMBO stent.
Limitations and strengths
First, the main limitation of these analyses is that the data are not randomised. The propensity score was calculated based on 13 pre-specified baseline and angiographic characteristics, but there could be other factors playing a role in clinical outcome that were not taken into account (e.g., chronic kidney disease and depressed left ventricular ejection fraction). Also, matching was performed with only limited angiographic characteristics. This is an important limitation that we acknowledge. The REMEDEE registry did not have core laboratory-adjudicated data on angiographic characteristics. Angiographic data were entered into the database by the sub-investigator of the site. All angiographic data in the DUTCH PEERS trial were core laboratory-adjudicated and obtained by means of quantitative coronary angiography (QCA; Medis, Leiden, the Netherlands). However, all events were adjudicated by an independent clinical events committee in the DUTCH PEERS trial and also in the REMEDEE registry. In addition, we could not correct for the fact that the patients treated in DUTCH PEERS were all patients in the Netherlands, and the REMEDEE registry consisted of European patients. The COMBO is compared with two different DES together (either Resolute Integrity or PROMUS Element). The results of the randomised DUTCH PEERS trial showed non-inferiority of Resolute Integrity to PROMUS Element, allowing these DES results to be pooled. For the main analysis we used the pooled set because of the increased power of a larger set of matched pairs. The separate analyses in Supplementary Table 1-Supplementary Table 5 show comparable outcomes24.
This analysis is the first to compare the COMBO stent with other newer-generation DES in a balanced cohort at two-year follow-up. Results of the study evaluating short (three months) versus standard (12 months) duration of DAPT in ACS patients, the REDUCE study, are awaited. Randomised data comparing the COMBO with the everolimus-eluting XIENCE stent (Abbott Vascular, Santa Clara, CA, USA) are expected from the forthcoming HARMONEE trial (NCT02073565).
Conclusions
In a propensity-matched cohort of patients treated with the novel COMBO stent and patients treated with either a PROMUS Element or Resolute Integrity DES, no differences were found in the clinical endpoint target lesion failure, with overall low adverse event rates. However, randomised trials are needed to demonstrate the equivalence of the COMBO stent to other current-generation DES.
| Impact on daily practice The dual-therapy stent technology has been evaluated in all-comers patients and has shown good clinical results. Currently, there are no data comparing the clinical results with other commonly used newer-generation DES, such as the PROMUS Element and Resolute Integrity. Two-year clinical follow-up of the COMBO stent shows low event rates, not significantly different from the PROMUS Element and Resolute Integrity stents, in a balanced cohort. Randomised controlled trials evaluating the clinical results after COMBO stent deployment are currently being conducted and their results are keenly anticipated. |
Acknowledgements
We acknowledge all patients for their participation in both studies and all interventional cardiologists and study nurses for conducting these studies. We especially thank all principal investigators of the DUTCH PEERS trial and REMEDEE registry: P. Danse, G. Jessurun, R. Hautvast, G.K. van Houwelingen, A.R. Schramm, R.M. Tjon Joe Gin, J.W. Louwerenburg, F.H.A.F. de Man, M.G. Stoel, G. Linssen, S.A.M. Saïd, M.B. Nienhuis, P.M.J. Verhorst, M.W.Z. Basalus, C.J.M. Doggen, K. Tandjung, P. den Heijer, I.B.A. Menown, A. Erglis, H. Suryapranata, K.E. Arkenbout, A. Iñiguez, A.W.J. van ’t Hof and P. Muller.
Funding
The AMC received an unrestricted research grant from OrbusNeich BV. Boston Scientific and Medtronic equally funded the DUTCH PEERS trial.
Conflict of interest statement
C. von Birgelen has received consultancy fees from Biotronik, Boston Scientific, and Medtronic, and institutional grant support from AstraZeneca, Biotronik, Boston Scientific, and Medtronic. R.J. de Winter has received consultancy fees from OrbusNeich and Abbott, and grant support from AstraZeneca, Stentys and Tryton Medical. The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Table 1A. PROMUS Element and Resolute Integrity and COMBO baseline characteristics before PS matching.
Supplementary Table 1B. PROMUS Element with Resolute Integrity and COMBO baseline characteristics before PS matching.
Supplementary Table 2. Resolute Integrity versus COMBO baseline characteristics after PS matching.
Supplementary Table 3. Clinical endpoints at two-year follow-up - COMBO versus Resolute Integrity.
Supplementary Table 4. PROMUS Element versus COMBO baseline characteristics after PS matching.
Supplementary Table 5. Clinical endpoints at two-year follow-up– COMBO versus PROMUS Element.

