
Introduction
The BuMA Supreme™ (SINOMED, Tianjin, China) is a sirolimus-eluting stent that has a biodegradable PLGA-polymer coating with an electro-grafting base layer on a thin-strut (80 µm) cobalt-chromium platform1. The PIONEER trial is a randomised study assessing the efficacy and safety of the BuMA Supreme compared with the Resolute™ stent (Medtronic, Minneapolis, MN, USA). The primary endpoint was the non-inferiority of the BuMA Supreme in terms of the nine-month angiographic in-stent late lumen loss (LLL). At nine months, the BuMA Supreme had a significantly higher in-stent LLL than the Resolute stent (0.29±0.33 vs 0.14±0.37 mm)1. Nevertheless, post hoc quantitative flow ratio (QFR) analysis showed no significant between-device difference in the value of QFR distal to the stent (0.89±0.10 vs 0.89±0.11, p=0.97) at nine months2. The aim of this report is to present the three-year clinical outcomes.
Methods
The study design and endpoint definitions have been described in a previous report1. All clinical events were adjudicated by an independent clinical events committee. Post hoc QFR analysis was performed in patients with clinically indicated (CI) target lesion revascularisation (TLR) or target vessel myocardial infarction (TVMI). The composite endpoint (device-oriented composite endpoint [DOCE]) was reported hierarchically. Time-to-event curves were constructed using the Kaplan-Meier estimates and compared by the log-rank test.
Results
A single three-year clinical follow-up visit was performed in 96.3% (BuMA) and 96.4% (Resolute) of the initial cohorts, p=1.00. Between one and three years, one CI target vessel revascularisation (CI-TVR) and one TVMI were adjudicated in a single patient in the BuMA group who presented with non-ST-elevation myocardial infarction (NSTEMI) and underwent revascularisation; there were no late events in the Resolute group. Notably, this revascularisation event in the BuMA group that converted an NSTEMI without topographic ECG changes into a CI-TVR and TVMI could not be justified by post hoc QFR analysis (Figure 1, Supplementary Table 1).
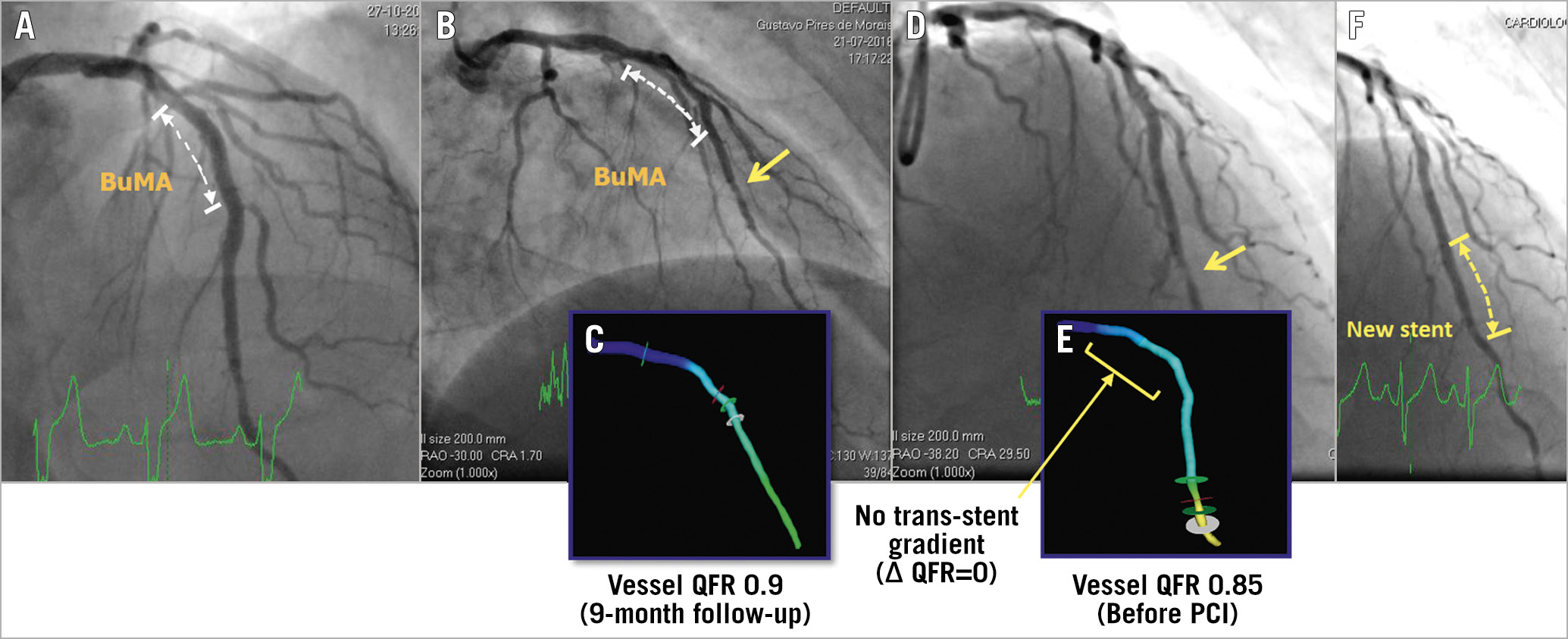
Figure 1. Case illustration. A) Post-index PCI procedure. BuMA Supreme was implanted. B) At nine-month angiographic follow-up. A discrete lesion of the distal left anterior descending artery (arrow). C) Off-line vessel QFR was 0.9. D) The patient presented with NSTEMI and received angiographic follow-up on day 915. E) Off-line vessel QFR was 0.85. F) A new stent was implanted on day 915. According to the clinical events committees, this NSTEMI can be counted as a TVMI since this event was not clearly attributable to a non-target vessel. Metastatic adenocarcinoma of the pancreas with hypercoagulability (a probable paraneoplastic syndrome) was diagnosed on day 1,044. The patient completed the three-year visit on day 1,164.
At three years, DOCE, a composite of cardiac death, TVMI and CI-TLR, was comparable between the groups (BuMA 7.2% vs Resolute 5.7%, log-rank p=0.72). The rates of cardiac death, TVMI and CI-TLR were similar (Table 1, Figure 2). Cumulative frequency distribution curves of vessel QFR at nine months are presented in Figure 3.
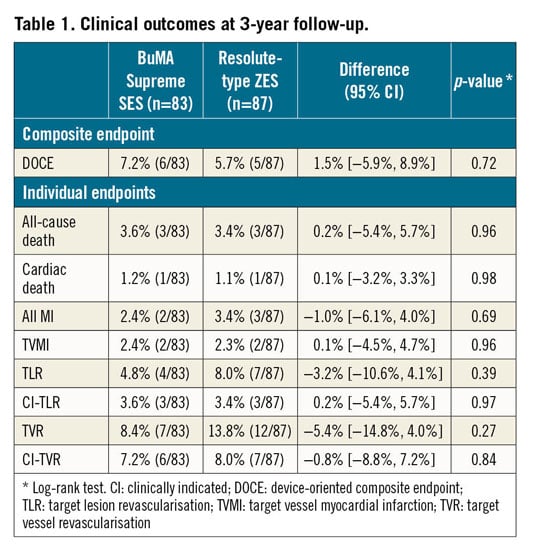
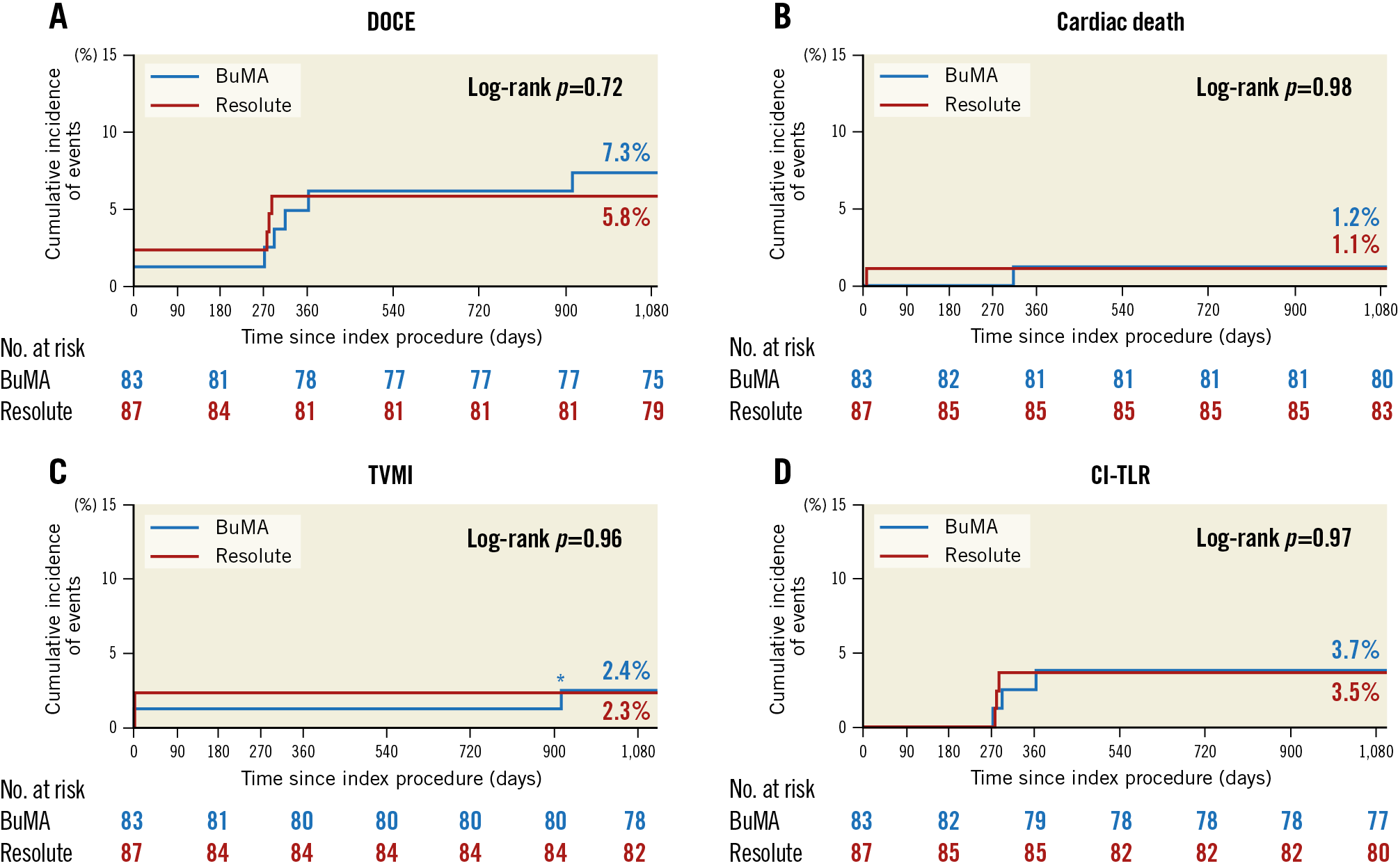
Figure 2. Kaplan-Meier curves for the clinical endpoints up to three-year follow-up. A) DOCE. B) Cardiac death. C) TVMI. D) CI-TLR. *TVMI at day 915, as reported in the text and the caption of Figure 1.
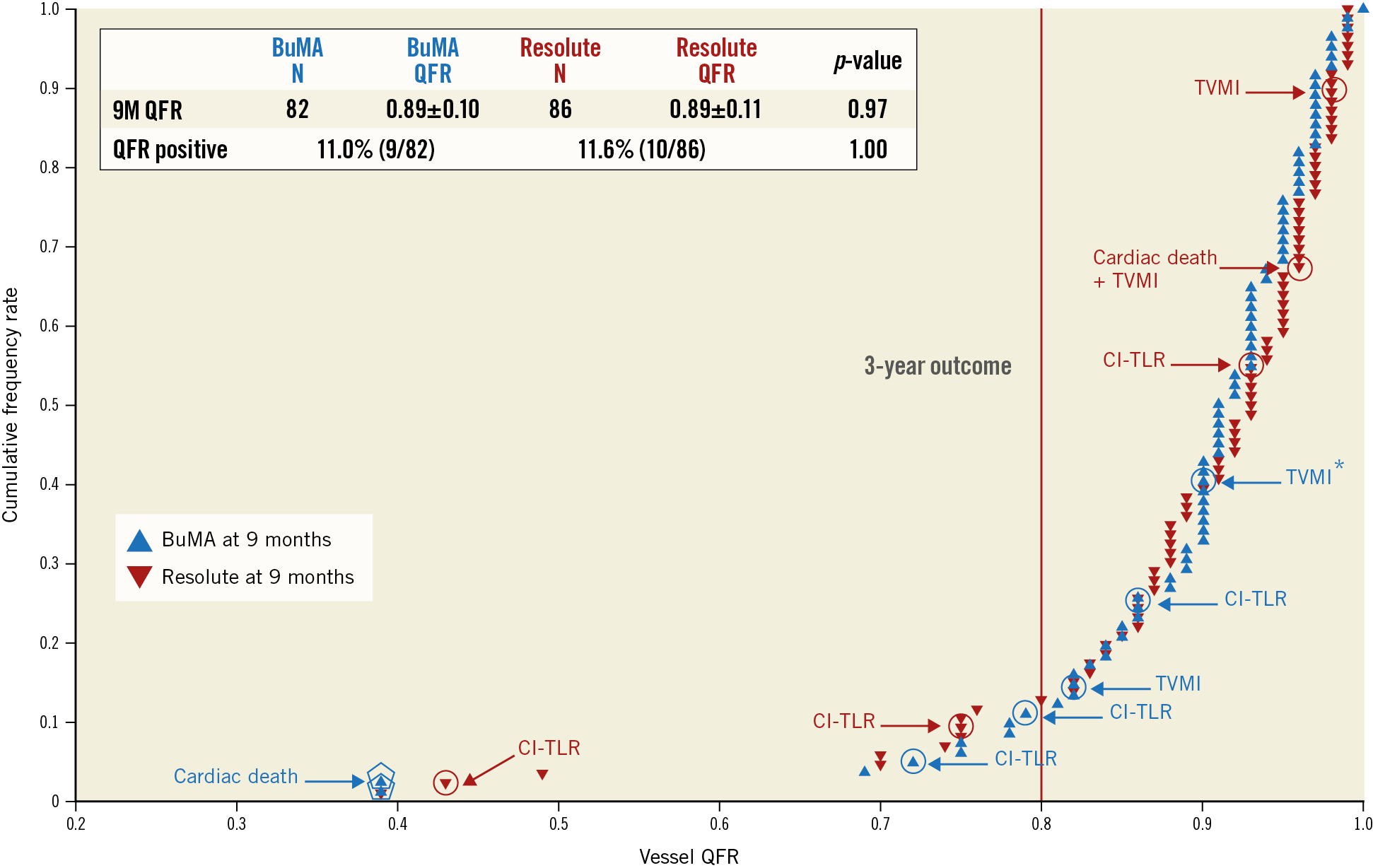
Figure 3. Cumulative frequency distribution curves of vessel QFR at nine months with intercurrent events up to three years. *TVMI at day 915, as reported in the text and the caption of Figure 1.
Discussion
Although the in-stent LLL of the BuMA Supreme was higher than that of the Resolute stent, this difference had no functional repercussions and no impact on TLR at three years. Moreover, the nine-month in-segment diameter stenosis did not differ between the groups (BuMA 24.5±12.4% vs Resolute 23.9±12.5%, p=0.74)1 and was in the range of the new drug-eluting stents as well as conforming to the objective performance index of coronary stents in Europe.
The impact of LLL on TLR has been evaluated in a meta-analysis of contemporary coronary devices3. The result demonstrated that the optimal cut-off value of LLL for predicting TLR was 0.50 mm. Low values of LLL may not serve as predictors of TLR.
Quantitative coronary angiography (QCA) is the most widely accepted method for assessing coronary devices and for endpoint adjudication in clinical trials. The recently published Academic Research Consortium-2 Consensus Document4 recommends the use of instantaneous wave-free ratio (iFR) and fractional flow reserve (FFR) for clinical endpoint adjudication. “Similar technologies, such as distal coronary pressure to aortic pressure ratio, contrast/saline FFR, QFR, and FFR from computed tomography, can be implemented for adjudication purposes with known limitations if specified in study protocol”. QFR has the potential to validate stent performance in a more clinically relevant fashion compared to QCA with less invasiveness than wire-derived functional assessment2. Nevertheless, further investigations are needed.
Limitations
The PIONEER trial was not powered for the comparison of clinical endpoints between the two groups. Moreover, the number of clinical events occurring from one- to three-year follow-up was very low in both groups. This observation may reflect a relatively low risk and low anatomic complexity population.
Conclusion
Despite a significant but subclinical difference in in-stent LLL at nine months, the three-year follow-up results demonstrated similar clinical outcomes between the BuMA Supreme and the Resolute stent.
|
Impact on daily practice In the PIONEER trial, although the BuMA Supreme had a higher angiographic in-stent late lumen loss compared to the Resolute stent at nine-month follow-up, functional assessments by QFR were similar. The use of angiography-derived functional assessment that does not require the use of additional catheters may play an increasing role in the assessment of device performance and would justify a revascularisation as clinically indicated. |
Guest Editor
This paper was guest edited by Adnan Kastrati, MD; Deutsches Herzzentrum, Munich, Germany.
Funding
This study was funded by SINOMED, Beijing, China.
Conflict of interest statement
C. von Birgelen reports institutional research grants to the research department of the Thoraxcentrum Twente, provided by Abbott Vascular, Biotronik, Boston Scientific and Medtronic, outside the submitted work. M. Sabaté reports grants and personal fees from Abbott Vascular, outside the submitted work. Y. Onuma was an advisory board member of Abbott Vascular. P.W. Serruys reports personal fees from Abbott Vascular, Boston Scientific, Biosensors, Medtronic, Philips/Volcano, Sinomedical Sciences Technology, SMT and Xeltis, outside the submitted work. The other authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

