
Abstract
Aims: In the PANDA III trial, the novel poly-lactide-co-glycolide polymer-based BuMA sirolimus-eluting stent (SES) was non-inferior to the polylactide polymer-based Excel SES for the primary endpoint of one-year target lesion failure (TLF), with a lower incidence of stent thrombosis. We sought to investigate whether the effectiveness profile of BuMA SES, with more rapid drug elution and polymer absorption kinetics, would persist at two years.
Methods and results: A total of 2,348 patients (mean age, 61.2±10.6 years; 24.3% diabetics; 31.2% with acute myocardial infarction within one month) were randomly assigned to receive either BuMA SES (n=1,174) or Excel SES (n=1,174) in the “all-comer” PANDA III trial. Two-year clinical follow-up was available for 2,262 (96.3%) patients. The incidence of TLF and the patient-oriented composite endpoint (PoCE) was low and similar between the BuMA and Excel groups (7.4% vs. 6.9%, p=0.67, and 13.1% vs. 10.9%, p=0.11, respectively). The rate of any revascularisation was significantly higher with the BuMA SES (6.8% vs. 4.6%, p=0.03). Definite and probable thrombosis occurred in 0.7% and 1.4% of patients in the BuMA and Excel groups, respectively (p=0.10).
Conclusions: Two-year rates of TLF and PoCE events were low and similar between the two biodegradable polymer-based SES. ClinicalTrials.gov Identifier: NCT02017275.
Abbreviations
BES: biolimus-eluting stent(s)
BP: biodegradable polymer
DES: drug-eluting stent(s)
DP: durable polymer
OCT: optical coherence tomography
PCI: percutaneous coronary intervention
PoCE: patient-oriented composite endpoint
SES: sirolimus-eluting stent(s)
ST: stent thrombosis
TLF: target lesion failure
Introduction
The advent of drug-eluting stents (DES) to address the limitations of bare metal stents is considered a significant milestone in percutaneous coronary intervention (PCI). However, late stent thrombosis (ST) and neoatherosclerosis have been observed with DES1-3 which, based on pathological evidence, appears most likely to be a consequence of delayed endothelial healing secondary to hypersensitivity reaction to the durable polymer (DP)4. Therefore, stents with a biodegradable polymer (BP) coating were developed with the intention of decreasing inflammatory reaction after full release of the drug, thereby overcoming the risk of a delayed healing process5. Previous studies have established similar safety and efficacy profiles of BP-DES compared with DP-DES6,7 and, in a recent meta-analysis including 16 randomised clinical trials, event rates at the longest available follow-up (mean 26 months) were similar for BP-DES and second-generation DP-DES8. However, the influence of the kinetics of polymer degradation or drug release of BP-DES has scarcely been studied.
To focus study of the underlying mechanisms of possible differences in clinical outcomes on the kinetics of polymer degradation and drug elution, clinical outcomes for two BP-DES, both sirolimus-eluting stents (SES) with the same platform material, namely the polylactide (PLA) polymer-based Excel stent (JW Medical Systems, Weihai, China) and the poly-lactide-co-glycolide (PLGA) polymer-based BuMA stent (Sino Medical, Tianjin, China), were compared in the multicentre, randomised controlled PANDA III trial (Comparison of BuMA Electro-grafting Based BioDegradable Polymer Stent With EXCEL Biodegradable Polymer Sirolimus-eluting Stent in “Real-World” Practice)9. At one year, the BuMA stent with more rapid drug elution and polymer absorption kinetics proved to be non-inferior to the Excel SES for the primary endpoint of target lesion failure (TLF), along with having a lower incidence of ST. The present study aimed to analyse the outcomes of the PANDA III all-comers population at two years, i.e., long after drug elution and polymer absorption had been completed for both stents.
Methods
STUDY DESIGN AND POPULATION
The study design and detailed methods of the PANDA III trial have been described previously9. Briefly, PANDA III was a randomised trial comparing two BP-based SES with different drug elution profiles and polymer biodegradation kinetics in an “all-comer” population. Eligible patients had at least one coronary artery stenosis greater than 50% with visually estimated reference diameter of 2.5 mm to 4.0 mm. Key exclusion criteria were limited to known allergy to contrast and/or device or study medications, and planned surgery within six months after the index procedure. There were no restrictions as to the lesions or vessels.
Ethics committees from each centre approved the study protocol, and all patients provided written informed consent. Patients were randomised in a 1:1 ratio to receive a BuMA or Excel stent using a web-based allocation system stratified by centre.
STUDY ENDPOINTS
The primary outcome of this study was TLF, a composite of cardiac death, target vessel myocardial infarction (MI), or ischaemia-driven target lesion revascularisation (TLR). Secondary endpoints included the patient-oriented composite endpoint (PoCE), a composite of all-cause death, all MI, or any revascularisation, TLF and PoCE components, and ST. Details of the endpoint definitions are as in the previous report9. Patients were followed up by telephone, and all adverse events were collected and recorded by the independent data safety and monitoring board (R&G, Beijing, China). An independent clinical events committee adjudicated all clinical events for analysis.
STUDY DEVICES AND PROCEDURES
The BuMA stent and Excel stent share the same platform material and eluting drug (Supplementary Table 1). Both devices have biodegradable polymer, differing in the unique design incorporating an electrografting (eG) base layer between the polymer and stent strut in the BuMA stent, which allows complete elution of sirolimus within 30 days and absorption of the PLGA polymer within three months. In contrast, the PLA polymer-based Excel stent elutes sirolimus completely within 180 days, and the PLA polymer is completely absorbed within six to nine months.
Stent implantation was performed according to standard techniques. Patients were treated with ≥100 mg of aspirin daily for an indefinite period and 75 mg of clopidogrel daily for at least 12 months.
STATISTICAL ANALYSIS
The sample size and power calculation for the study have been reported previously9. Data are presented as percentages and frequencies for dichotomous and categorical variables and were compared using the chi-square or Fisher’s exact test. Continuous variables are presented as mean±SD and were compared using the Student’s t-test. The 95% confidence intervals of the differences between the two treatment arms were calculated by normal approximation for continuous variables and by the Newcombe score method for binary variables. Kaplan-Meier analysis was used to calculate the time to clinical events, and the log-rank test to assess between-group differences. A landmark analysis at one year was performed for adverse clinical endpoints. Cox proportional hazards regression analysis was used to test for interaction between subgroups and stent type for the clinical endpoint of TLF. Multivariate Cox proportional hazards models were constructed to identify independent predictors of two-year TLF. All statistical analyses were performed using SAS software, version 9.1.3 (SAS Institute, Cary, NC, USA).
Results
A total of 2,348 patients were randomly assigned to treatment with BuMA SES (n=1,174) or Excel SES (n=1,174); 1,136 (96.8%) patients in the BuMA group and 1,126 (95.9%) patients in the Excel group completed follow-up at two years (Figure 1). Table 1 summarises the baseline demographics, and clinical and lesion characteristics of all patients.
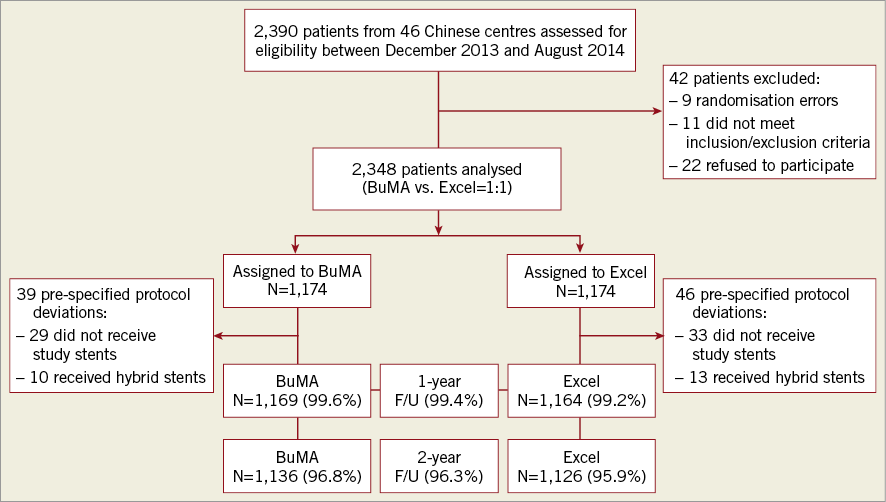
Figure 1. Patient flow chart. Two-year follow-up includes a window of ±30 days. The per-treatment evaluable population consisted of subjects who received only study device(s) at the target lesion and who had no pre-specified protocol deviations.
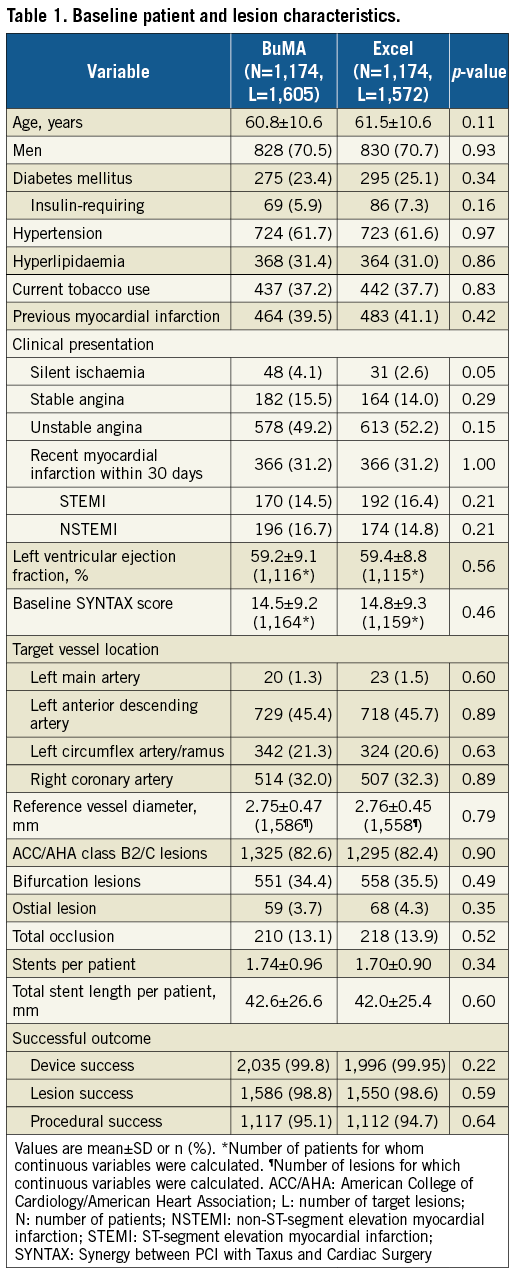
At two years, there were still no significant differences in TLF between the two arms, 7.4% in the BuMA group and 6.9% in the Excel group, with a p-value of 0.67 (Table 2, Figure 2, Supplementary Figure 1, Supplementary Table 2). The patient-oriented outcome was also comparable between the BuMA and Excel groups, 13.1% vs. 10.9%, respectively (p=0.11). In contrast, the incidence of any revascularisation was significantly higher in the BuMA group (6.8% vs. 4.6%, p=0.03), mainly driven by a numerically higher rate of ischaemia-driven target vessel revascularisation (TVR) and TLR events compared to the Excel group (3.2% vs. 2.0%, p=0.07, and 2.4% vs. 1.5%, p=0.14, respectively). All-cause death occurred in 39 patients in the BuMA group and 27 patients in the Excel group, without a significant difference. Myocardial infarction rates were 4.9% vs. 5.6%, respectively (p=0.48).
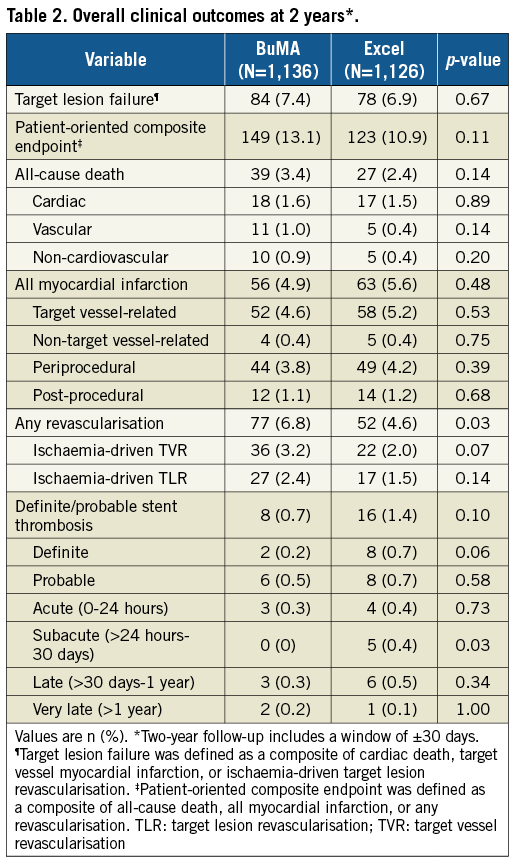
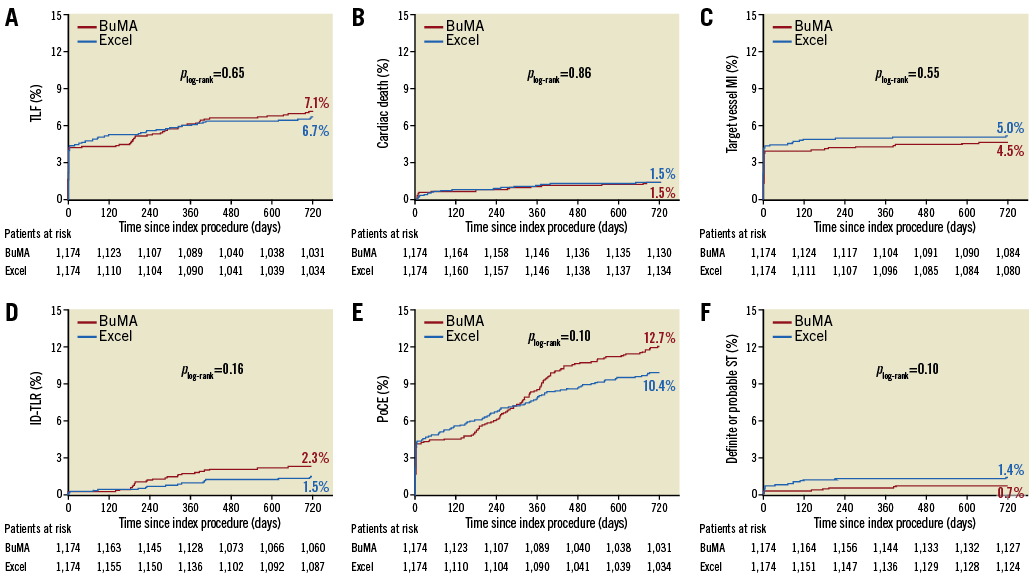
Figure 2. Kaplan-Meier curves for TLF and ST up to two years. Cumulative event curves up to two years for target lesion failure (A), cardiac death (B), target vessel myocardial infarction (C), ischaemia-driven target lesion revascularisation (D), patient-oriented composite endpoint (E), and definite or probable stent thrombosis (F) for patients receiving BuMA (red line) or Excel (blue line) stents are shown. ID-TLR: ischaemia-driven target lesion revascularisation; MI: myocardial infarction; PoCE: patient-oriented composite endpoint; ST: stent thrombosis; TLF: target lesion failure
The incidence of definite/probable stent thrombosis up to two years did not differ significantly, being 0.7% in the BuMA group and 1.4% in the Excel group (p=0.10). The rate of definite stent thrombosis was numerically lower for the BuMA group (BuMA vs. Excel: 0.2% vs. 0.7%, p=0.06). Very late stent thrombosis occurred in two (0.2%) versus one (0.1%) patient, respectively. At two-year follow-up, 38.7% (440 of 1,136) and 39.9% (449 of 1,126) of the surviving patients in the BuMA and Excel groups were still on dual antiplatelet therapy (DAPT) (Figure 3). We additionally analysed the correlation of two-year safety outcomes with DAPT cessation (Supplementary Table 3). The results showed that the incidence of spontaneous MI and definite/probable ST was similar between patients on and off DAPT.
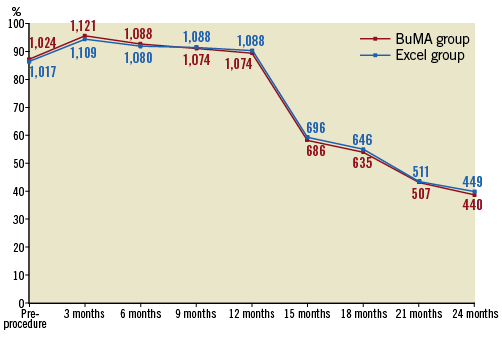
Figure 3. DAPT utilisation before and after the index procedure. The line chart shows the percentage of patients who continued DAPT; patient numbers are marked in the graph. DAPT: dual antiplatelet therapy
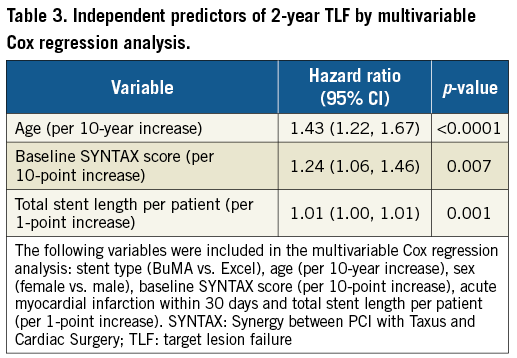
The pre-specified subgroup analysis revealed no significant between-stent difference in TLF at two years across subgroups except for bifurcation lesions (Figure 4). The results showed that, for patients with bifurcation lesions, Excel SES implantation might be associated with a decreased risk of TLF. In multivariable Cox regression analysis, age, baseline SYNTAX score, and total stent length per patient were independent predictors of two-year TLF (Table 3). Stent type was not an independent predictor of TLF.
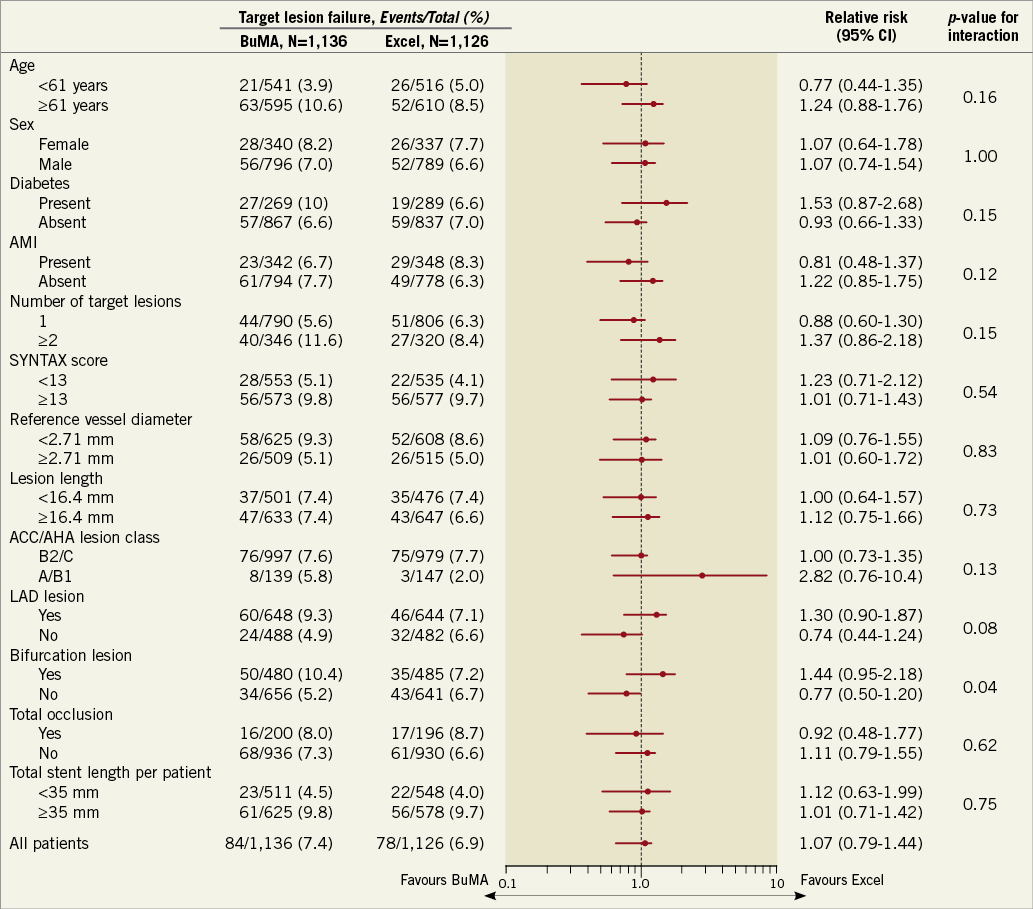
Figure 4. Subgroup analysis of target lesion failure at two years. The p-value for interaction represents the likelihood of interaction between the variable and the relative treatment effect. ACC: American College of Cardiology; AHA: American Heart Association; CI: confidence interval; DAPT: dual antiplatelet therapy; LAD: left anterior descending artery; SYNTAX: Synergy between PCI with Taxus and Cardiac Surgery
Discussion
The two-year results of the PANDA III trial showed that the incidence of TLF and PoCE was low and similar between the two groups, while event rates of any revascularisation were significantly higher in the BuMA group, mainly driven by a numerically higher incidence of ischaemia-driven TVR and TLR.
At one year, the BuMA SES with fast drug elution and polymer degradation kinetics was associated with similar safety and efficacy profiles but decreased rates of stent thrombosis events compared with the Excel SES9. As previously reported, both devices would have completed polymer absorption before one year, ending up with the same stainless steel platform. Although a numerically lower ST event rate was found at two years, driven mainly by subacute and late ST, the incidence of ST events at one to two years was similar between the two groups. The findings might support the theory that differences in polymer degradation and elution kinetics may influence early safety outcomes. After the absorption process is completed, the two stents would perform similarly. Results at two years also showed a non-significantly higher incidence of PoCE in the BuMA group, while rates of TLF and myocardial infarction were similar. The difference in PoCE was mainly driven by a higher event rate of TVR as well as TLR, which might raise concern on late efficacy issues of the BuMA stent with fast drug elution kinetics. However, the BIO-RESORT trial, comparing two BP-DES with very different polymer coating and degradation duration and a DP-ZES, documented similar safety and efficacy outcomes as well as comparable thrombosis rates at one year10. The strut thickness of the BP-DES in the PANDA III trial was 100-110 μm for BuMA SES and 120-130 μm for Excel SES, while BP-DES in the BIO-RESORT trial had very thin struts (60-80 μm). The incidence of TLF was 1%-2% higher in the PANDA III trial than in the BIO-RESORT trial, suggesting that, beyond drug elution and polymer degradation kinetics, strut thickness might be an important risk factor for long-term prognosis of BP-DES.
Studies have suggested better safety and efficacy profiles for the biodegradable polymer DES compared with early-generation DES11-13. In the final five-year follow-up of the LEADERS (Limus Eluted From A Durable Versus ERodable Stent Coating), BP biolimus-eluting stents (BES) were associated with a significant reduction in very late ST risk and related composite clinical outcomes14. In the pivotal randomised EVOLVE (A Prospective Randomized Multicenter Single-blind Non-inferiority Trial to Assess the Safety and Performance of the Evolution Everolimus-Eluting Monorail Coronary Stent System) II trial, the SYNERGY™ coronary stent was non-inferior to the predicate PROMUS Element™ Plus stent (both Boston Scientific, Marlborough, MA, USA) for one-year TLF15. Windecker et al demonstrated that, compared with a durable polymer stent, a biodegradable polymer-based stent was non-inferior for nine-month in-stent late lumen loss with comparable clinical event rates16. Similar results were reported for the CENTURY II (Clinical Evaluation of New Terumo Drug-Eluting Coronary Stent System in the Treatment of Patients with Coronary Artery Disease) trial17. In the three-year outcomes of the SORT OUT VI trial, a DP zotarolimus-eluting stent and a BP biolimus-eluting stent yielded similar safety and efficacy clinical outcomes, including stent thrombosis11. However, there are also studies indicating higher event rates for biodegradable polymer stents compared with DP-DES. In a large patient-level pooled analysis of the NEXT (NOBORI Biolimus-Eluting Versus XIENCE/PROMUS Everolimus-eluting Stent Trial) and COMPARE II (Abluminal biodegradable polymer biolimus-eluting stent versus durable polymer everolimus-eluting stent) studies, a higher rate of target vessel MI was observed in the BP-BES group18.
An optical coherence tomography (OCT) substudy showed that, at two years after stent implantation, stent struts not covered by neointima and evagination were less frequently observed in DP-DES compared with BP-BES (2.1±4.7% vs. 7.9±10.8% respectively, p=0.013)19. In terms of the present study, there is a prior single-centre, randomised OCT study that showed that the BuMA SES had superior strut coverage at three months compared with the Excel SES (94.2% vs. 90.0%, p<0.0001), with a relatively higher proportion of covered struts early at three months20, which we posited was related to lower thrombosis events at one year with BuMA SES. However, the influence was no longer significant at two years. As compared with DP-DES, BP-DES may provide an advantage by improving late/very late safety outcomes, mainly because durable polymer may contribute to delayed endothelial coverage and impaired stent healing. On the other hand, differing polymer absorption and drug elution profiles may influence the performance of BP-DES, especially at an early stage.
At one year, the incidence of TLF and especially ST was lower in the BuMA group in the acute myocardial infarction subgroup, while there was no between-group difference at two years, which might be secondary to the faster degradation kinetics of the BuMA SES, mainly benefiting the early healing process in patients. In this large-scale all-comer trial, patients appeared to derive little benefit from long-term DAPT use, which was consistent with the ITALIC trial21 but not the DAPT trial22. The optimal duration of DAPT with different types of drug-eluting stent remains a controversial topic, and whether DAPT duration should be shortened or prolonged in patients implanted with BP-DES warrants further investigation. In terms of two-year TLF, patients with bifurcation lesions tended to benefit more from the Excel SES. PCI for bifurcation lesions had been associated with worse clinical outcomes compared with non-bifurcation lesions, and BP-DES yielded at least comparable clinical prognosis compared with DP-DES after treatment of bifurcation lesions23. In the subgroup analysis of the LEADERS all-comers trial, BES was associated with comparable safety and superior efficacy when compared with CYPHER® SES (Cordis, Cardinal Health, Milpitas, CA, USA)24. Because the subgroup analysis in the present study is limited by the lack of detailed information on bifurcation lesions, such as Medina type, the tendency for bifurcation lesions to benefit from longer elution time of an antiproliferative drug must be regarded as hypothesis-generating.
Limitations
Our study should be interpreted in the light of the following limitations. First, the study was powered for the primary endpoint of TLF; therefore, it was underpowered to detect differences in the individual components of the composite endpoints as well as ST. Second, while all adverse events were adjudicated by an independent blinded clinical events committee, some adverse events, especially revascularisation, might be related to previously implanted stents and not the ones studied. Third, because the BuMA SES is available in diameters from 2.50 mm to 4.00 mm, the study was designed to include patients with an estimated reference diameter of 2.5 mm or greater. Fourth, the two-year event rates were relatively low, which might be partly explained by the relatively longer DAPT duration than that of the clinical routine guidance of one year. Finally, the findings presented should be considered hypothesis-generating and warrant further study.
Conclusions
While the differing polymer absorption and drug elution kinetics of the two BP-DES in the PANDA III trial appeared to influence their performance at an early stage, the two-year follow-up results underscored similar safety and efficacy profiles.
| Impact on daily practice The two-year follow-up of the PANDA III trial documented a low incidence of TLF and PoCE with both BP-DES, which might render BP-DES a favourable choice for PCI. Differing polymer absorption and drug elution profiles may influence the performance of BP-DES. |
Funding
The PANDA III trial was sponsored by Sino Medical, Tianjin, China.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Supplementary Figure 1. Landmark analysis at 1 year.
Supplementary Table 1. BuMA and Excel stent specifications.
Supplementary Table 2. Clinical outcomes at 1-2 years.
Supplementary Table 3. Correlation of 2-year safety outcomes with DAPT duration.
To read the full content of this article, please download the PDF.

