
Abstract
Aims: The aim of this study was to compare the strut coverage of the XIENCE stent with that of the BuMA Supreme sirolimus-eluting cobalt-chromium stent, which has a shorter drug elution, on optical coherence tomography (OCT) one or two months after implantation.
Methods and results: The PIONEER-II OCT trial was a multicentre, two-arm randomised trial, which comprised two cohorts: cohort-1 underwent an OCT imaging one month after coronary intervention (BuMA: 16 patients with 18 lesions, XIENCE: 15 patients with 17 lesions), whereas cohort-2 underwent OCT at two months (BuMA: 21 patients with 21 lesions, XIENCE: 23 patients with 28 lesions). The primary hypotheses were non-inferiority of the BuMA stent to the XIENCE stent in percent strut coverage at one month (cohort-1) or two months (cohort-2). In cohort-1, the BuMA stent was non-inferior to the XIENCE stent in terms of the strut coverage (83.8±10.4% for BuMA vs. 73.0±17.5% for XIENCE, pfor noninferiority <0.001), and was also significantly higher than the XIENCE (pfor superiority 0.037). In cohort-2, the BuMA stent was non-inferior to the XIENCE stent in OCT strut coverage (80.3±18.3% vs. 73.3±21.3%, pfor noninferiority 0.006, pfor superiority 0.24). Healing scores showed better healing in the BuMA stent in cohort-1 (32.36±21.59 vs. 54.88±34.65, p=0.027), whereas there was comparable healing between the BuMA and XIENCE stents in cohort-2 (39.86±37.77 vs. 53.75±42.84, p=0.25).
Conclusions: The BuMA Supreme had a faster coverage than the XIENCE at one month, presumably due to faster and shorter sirolimus elution. The difference in tissue coverage became less evident at two months. ClinicalTrials.gov Identifier: NCT02747329.
Abbreviations
%DS: percent diameter stenosis
BP: biodegradable polymer
CI: confidence interval
DAPT: dual antiplatelet therapy
DES: drug-eluting stent
HS: healing score
LLL: late lumen loss
MLD: minimum lumen diameter
OCT: optical coherence tomography
PBMA: poly(n-butyl methacrylate)
PLGA: poly(lactic-co-glycolic acid)
QCA: quantitative coronary angiography
RVD: reference vessel diameter
SES: sirolimus-eluting stent
ZES: zotarolimus-eluting stent
Introduction
The second generation of drug-eluting stents (DES) has emerged with more biocompatible durable polymer coatings on thin-strut stents that have shown a better safety than first-generation DES1,2. Biodegradable polymer (BP)-DES were developed to prevent polymer-related adverse effects3. The BuMA Supreme sirolimus-eluting stent (SES) (SINOMED, Tianjin, China) consists of a thin-strut (80 µm) cobalt-chromium platform with a sub-micrometre-thin electro-grafted base layer, top-coated with a biodegradable coating that releases sirolimus relatively fast within 28 days. With the short drug elution time, the first-in-man trial of the BuMA Supreme stent showed a greater nine-month angiographic late loss of 0.29±0.33 mm as compared to 0.14±0.37 mm in the Resolute™ zotarolimus-eluting stent (ZES) (Medtronic, Minneapolis, MN, USA), although the additional analysis showed the absence of difference in quantitative angiography-derived FFR between the two devices4,5. This moderate late loss value could be the result of an early coverage of the BuMA Supreme stent.
The degradation of the top-layer poly-glyco-lactide acid coating of the BuMA Supreme stent is complete in six weeks6. To date, the advantage of BP-DES has been debatable. Some studies have shown improvement of clinical outcomes with BP-DES as compared to durable polymer DES, but the number of those studies is limited3,7. Recent studies have reported less advantage of BP-DES compared with second-generation DES with durable polymer8,9.
We hypothesised that the BuMA Supreme stent with its short elution time could achieve earlier strut coverage than the XIENCE stent (Abbott Vascular, Santa Clara, CA, USA) which has a relatively longer antiproliferative drug elution and a durable polymer coating. In this PIONEER-II OCT randomised trial comparing BuMA and XIENCE, the strut coverage was assessed on optical coherence tomography (OCT) one and two months after implantation.
Methods
The PIONEER-II OCT trial was a multicentre, two-arm randomised trial conducted in China, which comprised two cohorts: cohort-1 underwent an OCT imaging one month after coronary intervention, whereas cohort-2 underwent OCT at two months. In the current trial, 31 patients were assigned to cohort-1 (BuMA: n=16, XIENCE: n=15), whereas 44 patients were enrolled to cohort-2 (BuMA: n=21, XIENCE: n=23). The current OCT study was also designed to explore the early-phase strut coverage in the patients at high bleeding risk. Details of the eligibility criteria of the current study are presented in Supplementary Appendix 1. Briefly, the inclusion criteria were: (1) age 18-85; (2) presence of stable or unstable angina or silent ischaemia; (3) presence of one or two separate de novo target lesions (a single target lesion per major epicardial territory) with a 50-99% lumen diameter stenosis in a reference vessel of 2.5-4.5 mm (visually determined), and a target lesion length of <40 mm, and (4) presence of one or more high bleeding risks such as a history of prior intracerebral bleeding, presence of renal failure (calculated creatinine clearance <40 ml/min) or presence of non-skin cancer diagnosed or treated ≤3 years prior to the enrolment.
Eligible patients were randomly assigned to each cohort (cohort 1: one-month OCT follow-up or cohort 2: two-month OCT follow-up) and assigned to receive the BuMA Supreme SES or XIENCE everolimus-eluting stent family (the control device) in a 1:1 ratio in random blocks, stratified by centre using a web-based allocation system. The current study complied with the Declaration of Helsinki. Medical ethics committees at each participating institution approved the trial, and all patients provided a written informed consent.
STUDY DEVICES AND IMPLANTATION PROCEDURE
The BuMA Supreme SES is a DES with a rapid exchange balloon-expandable catheter delivery system. The device consists of an extremely thin (100-200 nm) electrografted (eG) base layer of poly(n-butyl methacrylate) (PBMA), covalently bound to an electronically polished thin-strut (80 µ) cobalt-chromium stent platform. The base layer’s molecules are on their other side connected by interdigitation with a 3.8 to 10 μm-thin top layer – a blend of a biodegradable poly(lactic-co-glycolic acid) (PLGA) polymer and sirolimus. This base layer prevents the bioactive coating from cracking and delamination during delivery and expansion of the stent6. Unlike other DES, the BuMA Supreme has a fast drug elution profile: it elutes 92% of sirolimus in the first 28 days and 100% at two months (Figure 1)7. The XIENCE family of stents was used in the control group. These DES are made from cobalt-chromium (81 µm) with a permanent fluoropolymer eluting everolimus10.
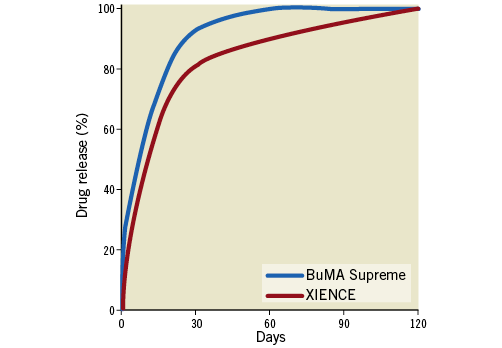
Figure 1. Drug release curves of the BuMA Supreme and XIENCE stents.
The interventional procedure was performed and dual antiplatelet therapy (DAPT) was given according to current clinical guidelines11. The patients underwent repeat angiography and OCT at either one month (cohort-1) or two months (cohort-2).
QUANTITATIVE CORONARY ANGIOGRAPHY (QCA)
Off-line QCA analyses were performed by an independent core laboratory (Cardialysis BV, Rotterdam, the Netherlands) with the CAAS system (Pie Medical Imaging, Maastricht, the Netherlands) according to standard operational protocols12. The following QCA parameters were calculated both in-stent and in-segment (including the 5 mm proximal and distal stent margins): minimum lumen diameter (MLD), interpolated reference vessel diameter (RVD), percent diameter stenosis (%DS: [1-MLD/RVD]×100), acute lumen gain (difference in MLD between pre-procedure and post-procedure) and late lumen loss (LLL: difference in MLD between post-procedure and follow-up).
OCT ANALYSIS
OCT assessment of the stented coronary segment was performed using the final OCT recordings sent to an independent core laboratory (Cardialysis BV) for off-line analysis using QIvus software, version 3.0 (Medis, Leiden, the Netherlands). A covered strut was defined as having a neointimal thickness >0 μm13. For the secondary endpoint analysis, stent area and derived measures were based on the endoluminal stent contour14. To quantify the degree of vascular healing status, the healing score (HS) was calculated according to the previously published methods15. Briefly, the HS was calculated by summing up the points from four components of OCT findings with specific weights: presence of intraluminal mass (assigned a weight of 4); presence of both malapposed and uncovered struts (assigned a weight of 3); presence of uncovered struts alone (assigned a weight of 2); and presence of malapposed struts alone (assigned a weight of 1).
ENDPOINTS
The primary study endpoint of this imaging study was the percentage strut coverage on OCT. There were two independent hypotheses for each cohort, which were: 1) for cohort-1, non-inferiority of the BuMA Supreme stent to the XIENCE stent for the primary endpoint at one month; 2) for cohort-2, non-inferiority of the BuMA Supreme stent to the XIENCE stent for the primary endpoint at two months. OCT imaging endpoints included the mean and minimal scaffold/stent and diameter, area and volume, the frequency of incomplete strut apposition including area and volume, the percentage of malapposed struts, the mean neointima thickness and neointimal hyperplasia area on top of the strut, and volume, the mean flow area and volume, the malapposition area and volume, and intraluminal defect area and volume14.
STATISTICAL METHODS AND SAMPLE SIZE CALCULATION
For binary variables, counts, percentages, and 95% confidence intervals (CI) were calculated. Pearson’s chi-squared test or Fisher’s exact test was performed when appropriate. For continuous variables, means, standard deviations, and 95% CI for the mean were calculated and compared with a t-test. A two-sided p-value <0.05 was considered statistically significant. All statistical analyses were performed using SAS version 9.3 (SAS Institute Inc, Cary, NC, USA) and SPSS, Version 24.0.0 (IBM Corp., Armonk, NY, USA).
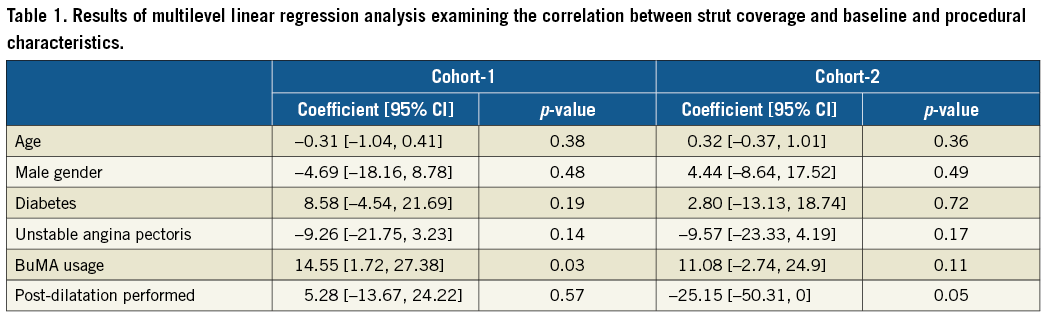
Multilevel linear regression analyses with random intercept and fixed slope model were used in order to investigate the impact of the usage of the BuMA stent on percentage of strut coverage while adjusting for baseline and procedural characteristics selected based on previously known determinants associated with neointimal hyperplasia or strut coverage (diabetes, unstable angina pectoris, post-dilatation)16-20. In these multilevel models, lesions were clustered within a patient when the patient had multiple lesions treated.
For cohort-1, the sample size was based on the following assumptions: based on the previous literature, the coverage rate of the XIENCE at one month was 70% and the BuMA 71.6%21. With a pooled standard deviation of 19%, one-sided alpha of 0.05 and a non-inferiority margin of 15%, 20 OCT per arm were needed to prove non-inferiority of BuMA to XIENCE with an 85% power. For cohort-2, the coverage rate of XIENCE and BuMA at two months was assumed to be 83% and 86.4%, respectively22,23. With a pooled standard deviation of 11%, one-sided alpha of 0.05 and a non-inferiority margin of 8.5%, 20 OCT per arm were required to prove non-inferiority of BuMA to XIENCE with a 95% power.
Results
From May 2016 to March 2017, 75 patients were recruited at eight national sites in China. The baseline characteristics of the two cohorts are presented in Supplementary Table 1. There were no significant differences in the two device groups, though there existed a non-significant trend towards higher incidence of diabetes in the XIENCE arm of cohort-2. The patient flow chart is shown in Figure 2. In cohort-1, all patients received DAPT at one-month follow-up in both arms. In cohort-2, 90.5% (19/21) of the patients in the BuMA arm received DAPT at two-month follow-up while all patients received DAPT in the XIENCE arm. All patients enrolled in the current study continued statin at follow-up. Details of the bleeding propensity of the enrolled patients are presented in Supplementary Table 2.
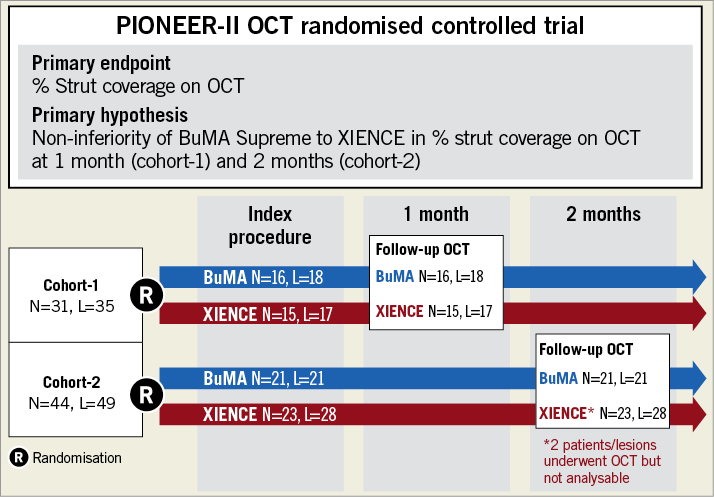
Figure 2. Study flow chart of the PIONEER-II OCT randomised controlled trial. L: number of lesions; N: number of patients
QCA AND OCT RESULTS AT ONE MONTH (COHORT-1)
The results of angiographic and OCT analysis are presented in Supplementary Table 3 and Supplementary Table 4, respectively. Post-procedural QCA results were comparable between the two groups. At one month, there was no binary restenosis and the in-stent LLL of the two devices was not statistically different (0.12±0.25 mm for BuMA vs. 0.03±0.20 mm for XIENCE, p=0.25).
On OCT at one month, the BuMA Supreme stent was non-inferior to the XIENCE stent in terms of strut coverage as assessed on OCT (83.8±10.4% for BuMA vs. 73.0±17.5% for XIENCE, pfor noninferiority <0.001), whereas the one-month OCT coverage rate of the BuMA Supreme was significantly higher than the XIENCE (pfor superiority 0.037) (Figure 3A). Mean neointimal hyperplasia area was greater in the BuMA than in the XIENCE (0.63±0.37 mm2 vs. 0.36±0.12 mm2, p=0.008) as was volume obstruction (7.5±4.9% vs. 4.9±1.7%, p=0.045). The healing score was lower in the BuMA than in the XIENCE stent (32.36±21.59 vs. 54.88±34.65, p=0.027), suggesting earlier healing with the BuMA in terms of coverage and malapposition (Figure 4A). Representative cases in cohort-1 are shown in Figure 5A. The quantitative parameters did not differ between the two arms: minimum lumen area was 6.43±1.39 mm2 and 5.80±2.12 mm2 in the BuMA arm and the XIENCE arm, respectively (p=0.30).
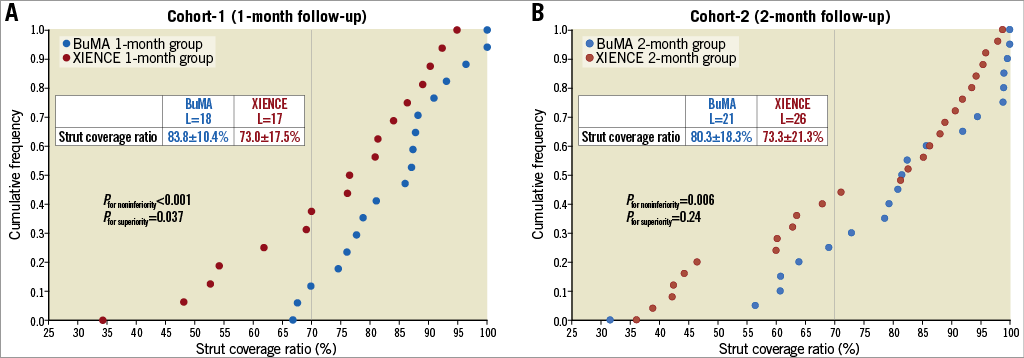
Figure 3. Cumulative frequency distribution curves of strut coverage as assessed on OCT in cohort-1 (A) and cohort-2 (B). L: number of lesions
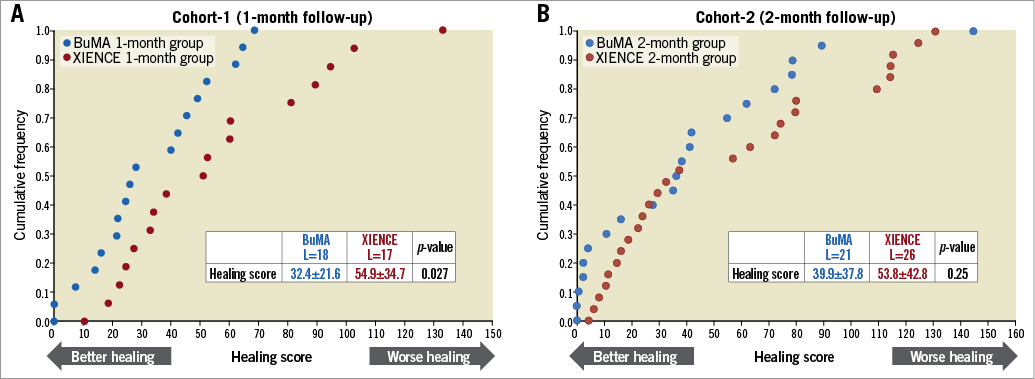
Figure 4. Cumulative frequency distribution curves of healing score as assessed on OCT in cohort-1 (A) and cohort-2 (B). L: number of lesions
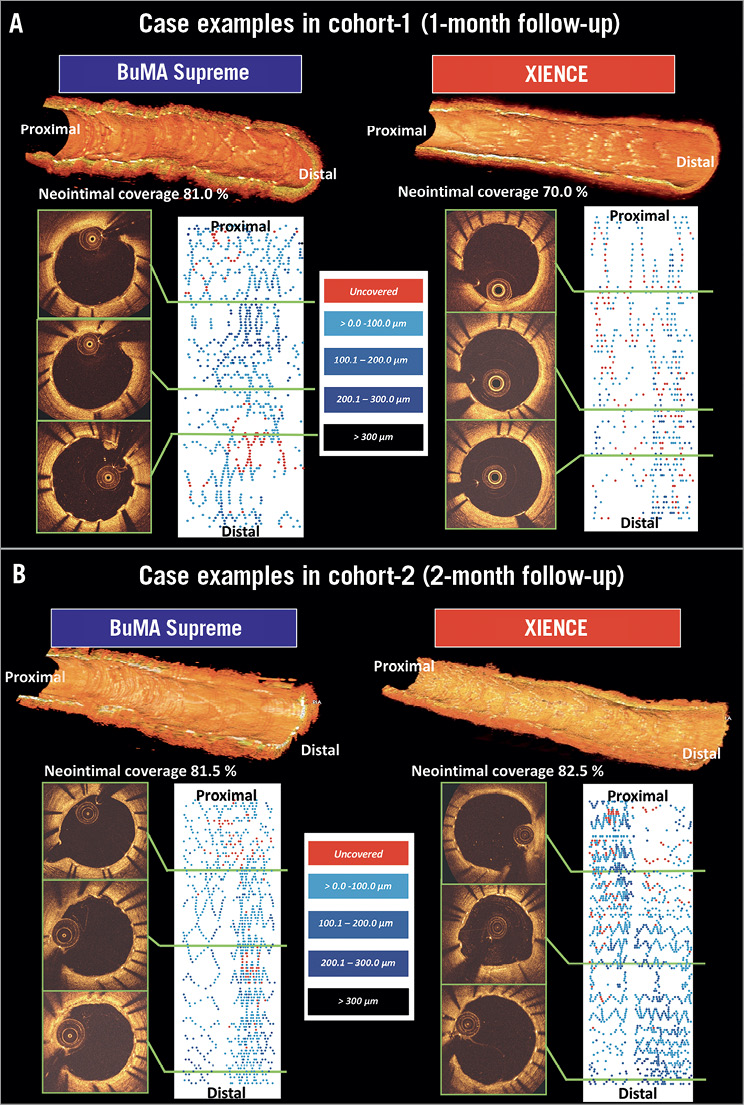
Figure 5. Representative cases in the PIONEER-II OCT randomised controlled trial. A) OCT in cohort-1. B) OCT in cohort-2. Three-dimensional (3D) reconstructions of OCT pullbacks of the representative cases in the BuMA and the XIENCE arms (upper). Cross-sectional images and 2D fold-out views of strut coverage of the same cases, in which the neointimal thickness on struts is presented with colour coding (lower).
QCA AND OCT AT TWO MONTHS (COHORT-2)
Quantitative assessment of angiography and OCT is shown in Supplementary Table 3 and Supplementary Table 4, respectively. Angiographic LLL at two months was 0.06±0.21 mm in the BuMA arm and 0.06±0.22 mm in the XIENCE arm. There was no binary restenosis. On OCT at two months, the BuMA Supreme stent was non-inferior to the XIENCE stent in OCT strut coverage (80.3±18.3% for BuMA vs. 73.3±21.3% for XIENCE, pfor noninferiority 0.006, pfor superiority 0.24) (Figure 3B). Healing score was comparable between the two groups (39.86±37.77 vs. 53.75±42.84, p=0.25) (Figure 4B). Representative cases in cohort-1 are presented in Figure 5B. The quantitative parameters did not differ between the two arms: minimum lumen area was 6.47±2.51 mm2 and 6.78±2.26 mm2 in the BuMA arm and the XIENCE arm, respectively (p=0.65).
MULTILEVEL LINEAR REGRESSION ANALYSIS INVESTIGATING THE IMPACT OF THE BuMA STENT ON STRUT COVERAGE
The results of the regression analyses are summarised in Table 1. In cohort-1, usage of the BuMA stent was independently associated with an increased percentage of strut coverage at one month (estimated coefficient: 14.55 [95% CI: 1.72 to 27.38]) while post-dilatation was not. In cohort-2, usage of the BuMA stent was not associated with the percentage of strut coverage. Although post-dilatation has a tendency towards a negative effect on the percentage of strut coverage at two months, there was no statistical significance (estimated coefficient: –25.15 [95% CI: –50.31 to 0]).
Discussion
POTENTIAL ADVANTAGE OF EARLY STRUT COVERAGE
The PIONEER-II OCT randomised controlled trial results suggest that the BuMA Supreme stent has faster coverage at an earlier time point (one month) than the XIENCE stent, whereas the difference in tissue coverage becomes less evident at a later time point (two months).
In human post-mortem histological analysis, it was reported that strut coverage was an independent predictor for late (>30 days) stent thrombosis24. In the current trial, most of the BuMA stent could reach sufficient strut coverage at the early time point. In cohort-1, the lesions with uncovered struts >30% were 16.7% (3/18) in the BuMA arm as opposed to 41.2% (7/17) in the XIENCE arm one month after implantation (p-value 0.15). In cohort-2, the lesions with uncovered struts >30% were 28.6% (6/21) in the BuMA arm as opposed to 42.3% (11/26) in the XIENCE arm two months after implantation (p-value 0.33).
THE ROLE OF FAST DRUG ELUTION AND POLYMER DEGRADATION
Generally, the technological determining factors of strut coverage after DES implantation have been related to the strut (its thickness), to the coating polymer (its biocompatibility or degradation process) and to the drug (release kinetics)25-27. The faster coverage of the BuMA Supreme stent could be mainly due to the faster drug release of sirolimus. The strut thickness of the BuMA stent is comparable to that of the XIENCE stent (80 µm for the BuMA stent and 81 µm for the XIENCE stent)6,10. The duration of complete polymer degradation of the BuMA stent is approximately eight weeks, which is shorter than other biodegradable polymer-coated stents6. This technological aspect may play a role during the study phase (two months) but obviously acts longer than two months after implantation. The BuMA stent releases the drug relatively rapidly so that the release of sirolimus from the stent is nearly complete by 28 days after implantation, whereas the drug release of the XIENCE stent continues until 120 days after stenting (Figure 1)7,28 The drug concentration in the polymer coating of the BuMA stent is 120 µm/cm2 while that of the XIENCE stent is 100 µm/cm2, suggesting that the BuMA stent elutes more concentrated cytostatic drug in a shorter time4,10. Nevertheless, this aspect probably did not impact on the early-phase neointimal healing in the current OCT study.
The fast strut coverage due to the modestly shorter drug elution of the BuMA stent has the potential to reduce the risk of stent thrombosis while lowering the risk of excessive luminal loss. The concept of fast drug elution was previously tested with the Endeavor® zotarolimus-eluting stent (E-ZES) (Medtronic). The E-ZES was designed to release the drug rapidly within 10 days and demonstrated a lower incidence of safety endpoints (stent thrombosis) and comparable efficacy endpoints (target lesion revascularisation) to first-generation DES (sirolimus-eluting stent and paclitaxel-eluting stent)29,30. Regardless of the favourable clinical outcomes of E-ZES, this technology was replaced by the Resolute zotarolimus-eluting stent (R-ZES) with long-lasting drug elution, possibly because of the efficacy concern derived from angiographic results: the E-ZES generated a higher angiographic LLL and binary restenosis rate than other DES, even though the clinical outcomes were comparable29. The BuMA Supreme has the same concept but the drug elution of BuMA is slower than that of E-ZES (28 days vs. 10 days). The difference in drug release between BuMA and R-ZES/E-ZES is in line with the results of angiographic in-stent LLL of each device in first-in-man trials (BuMA: 0.29±0.33 mm at nine months, R-ZES: 0.14±0.37 mm at nine months and E-ZES: 0.61±0.44 mm at 12 months), suggesting that the inhibition capability of neointimal proliferation of the BuMA stent is intermediate between R-ZES and E-ZES4,31. The faster strut coverage could be an advantage of the BuMA stent unless the neointimal hyperplasia is excessive up to the point of creating luminal obstruction in a later phase. Further studies, such as an OCT study with late-phase quantitative and qualitative neointimal assessment after BuMA implantation, are warranted in a larger population to investigate further the benefit of their initial faster strut coverage.
POTENTIAL IMPACT OF BASELINE PROPENSITY ON NEOINTIMAL PROLIFERATION
In the current trial, the percentage of diabetic patients was numerically higher in the XIENCE arm in both cohorts (cohort-1: 18.8% [3/16] for BuMA vs. 46.7% [7/15] for XIENCE, p-value 0.14; cohort-2: 14.3% [3/21] vs. 39.1% [9/23], p-value 0.06) at baseline. Diabetes is known to be associated with greater and faster neointimal hyperplasia compared to non-diabetes16,17. This is considered to be due to the hormonal and vascular abnormalities which promote smooth muscle cell proliferation after vascular injury16. The difference in the percentage of diabetic patients between the two arms might have influenced the results of the OCT study, even though this difference affects strut coverage in favour of the XIENCE arm.
Implantation technique can influence the neointimal response and strut coverage. In the current study, the percentage of lesions with post-dilatation in the BuMA arm was higher than that in the XIENCE arm (cohort-1: 100% [18/18] for BuMA vs. 76.5% [13/18] for XIENCE, p=0.045; cohort-2: 95.2% [20/21] vs. 85.7% [24/28], p=0.38) (Supplementary Table 1). Post-dilatation can be associated with better strut apposition and, furthermore, with greater expansion of the vessel wall and deeper embedment of struts into the vessel wall, but can induce vessel injury followed by increased neointimal hyperplasia19,20. Thus, post-dilatation may result in a good strut coverage in a late phase. More frequent post-dilatation in the BuMA arm in the one-month cohort might have had a favourable influence for the BuMA arm in terms of neointimal coverage. In the current study, these factors were not independent predictors of strut coverage either one or two months after implantation, but a potential impact may not be completely excluded.
Furthermore, patients with high bleeding risk were included in the current trial. Mainly, the patients had a history of cerebrovascular disease (ischaemic and haemorrhagic) (Supplementary Table 2). To the best of our knowledge, there are no public domain data with regard to the relationship between bleeding propensity and early-phase neointimal proliferation after stenting.
Limitations
A limitation of the study is its small sample size and non-inferiority design. Strut coverage was assessed by visual assessment by experienced core lab analysts, which may have a limited reproducibility32. The current study did not have OCT assessments at baseline. Vessel healing with neointimal coverage depends on the underlying lesion morphology. Neointimal qualitative assessment was not performed because at one or two months very thin neointima precluded precise assessment of the neointimal quality, even using OCT-based quantitative tissue characterisation software33. Non-significant trends in baseline characteristics could have confounded the results: for example, 1) diabetes rates were numerically higher in the XIENCE arm, which could have influenced the strut tissue coverage in favour of XIENCE; 2) post-dilatation rates were numerically higher in the BuMA arm, which could have affected the strut tissue coverage. Lastly, the non-serial design does not allow assessment of serial changes of coverage over time.
Conclusions
The results of the PIONEER-II OCT randomised controlled trial suggest that the BuMA Supreme stent has a faster coverage at a very early time point (one month) than the XIENCE stent, presumably due to faster sirolimus elution. The difference in tissue coverage became less evident at a later time point (two months).
| Impact on daily practice Fast strut tissue coverage after implantation has the potential to reduce the risk of stent thrombosis. In the current OCT study, the BuMA Supreme stent demonstrated better strut coverage compared with the XIENCE stent at one month. Taking advantage of the fast strut coverage, an abbreviated duration of dual antiplatelet therapy may be applied to patients treated with the BuMA Supreme stent. |
Guest Editor
This paper was guest edited by Antonio Colombo, MD, FACC, FESC, FSCAI; Department of Interventional Cardiology, EMO GVM Centro Cuore Columbus, Milan, Italy.
Acknowledgements
This manuscript was generated mainly by the contribution of the two cooperative teams. The Chinese PLA general hospital team was responsible for the design and execution of the project. Cardialysis was responsible for the core lab analysis and its academic team drafted the manuscript.
Funding
This study was funded by SINOMED, Tianjin, China.
Conflict of interest statement
P.W. Serruys is a member of the Advisory Board for SINOMED. The other authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.
Supplementary data
Supplementary Appendix 1. Study eligibility criteria.
Supplementary Table 1. Baseline characteristics.
Supplementary Table 2. Bleeding propensity of the enrolled patients.
Supplementary Table 3. Results of quantitative coronary angiography.
Supplementary Table 4. Results of optical coherence tomography.
To read the full content of this article, please download the PDF.

