Abstract
Aims: We performed a randomised controlled open-label non-inferiority trial to compare angiographic outcomes between the ultra-thin strut, biodegradable hybrid polymer Orsiro sirolimus-eluting stent (O-SES) and the durable biocompatible polymer Resolute Integrity zotarolimus-eluting stent (R-ZES).
Methods and results: A total of 372 patients planned to undergo percutaneous coronary revascularisation were randomly assigned 2:1 to treatment with O-SES or R-ZES (250 and 122 patients, respectively). O-SES was non-inferior to R-ZES for the primary endpoint, in-stent late lumen loss at nine months (median 0.06 mm [interquartile range, –0.09 to 0.24 mm] versus 0.12 mm [–0.07 to 0.32 mm]; p for non-inferiority <0.001; p for superiority=0.205). Percent diameter stenosis was significantly lower in the O-SES group than in the R-ZES group (15.0 [10.0 to 20.0] versus 20.0 [13.3 to 26.0]; p=0.002). Target lesion failure occurred in 2.4% and 3.3% of the O-SES and R-ZES groups, respectively (p=0.621). Subgroup analyses showed consistently similar outcomes between the two groups in terms of the primary endpoint, except for the diabetic subgroup.
Conclusions: O-SES was non-inferior to R-ZES in terms of in-stent late loss at nine months. Angiographic restenosis and clinical adverse events were low in both groups. This study confirms the good safety and efficacy profiles of both contemporary coronary stents.
Introduction
Drug-eluting stents (DES) have become an indispensable component in percutaneous coronary revascularisation1,2. Although the advent of DES has reduced the need for repeat revascularisation, concerns have been raised as studies have reported an increased propensity for very late stent thrombosis with DES use as compared to bare metal stents (BMS)3-5. This has provoked numerous innovations in DES design, for example changes in the polymer composition. As studies have suggested polymer as a culprit for thrombogenicity6,7, biocompatible durable polymers (DP) and biodegradable polymers (BP) have replaced previous polymers. Another innovation is thinner strut devices. Recent evidence suggests that the safety profile of a coronary stent is determined not only by the property of the polymers, but by an optimal combination of stent geometry, strut thickness, polymer characteristics, and antiproliferative drugs8.
The safety profile of earlier models of BP-DES was not as good as expected. The rate of stent thrombosis of BP biolimus-eluting stents was lower than that of first-generation DES, but higher than that of everolimus-eluting stents (EES), which is a second generation of DP-DES9,10. The Orsiro biodegradable polymer sirolimus-eluting stent (O-SES; Biotronik AG, Bulach, Switzerland) is a novel DES with an ultra-thin strut. Its hybrid coating ensures degradation of the biodegradable poly-L lactic acid polymer and blockade of metallic surface exposure to the surrounding tissue. O-SES has the thinnest strut thickness to date (60 μm), and thus provides good flexibility and deliverability. Previous studies have shown promising angiographic and clinical outcomes after implantation of O-SES11-13.
The Resolute Integrity® zotarolimus-eluting stent (R-ZES; Medtronic Cardiovascular, Santa Rosa, CA, USA) is one of the most widely used contemporary DP-DES. The RESOLUTE All Comers trial showed equivalent outcomes of the Endeavor Resolute ZES, a previous version of R-ZES, with the XIENCE V® everolimus-eluting stent (Abbott Vascular, Santa Clara, CA, USA)14. In addition, recent studies have shown good performance of R-ZES15,16. In this study, we performed a randomised controlled trial comparing angiographic outcomes of O-SES with the R-ZES in subjects undergoing percutaneous coronary intervention (PCI) for coronary artery disease. This study was an all-comers trial with limited exclusion criteria.
Methods
STUDY DESIGN
The Orsiro Hybrid sirolimus-eluting stent and Resolute Integrity zotarolimus-eluting stent in all-comers with coronary artery disease (ORIENT) trial is a prospective randomised open-label multicentre trial. The study design has been described previously17. The study participants were enrolled in eight centres in the Republic of Korea between October 2013 and June 2014. This trial was initiated by investigators, and grant support was provided by BIOTRONIK Korea Co., Ltd., Seoul, Republic of Korea. Data were managed by a contract research organisation (T&W software, Seoul, Republic of Korea). The data analysis was performed by the investigators. The authors are solely responsible for the design and execution of the trial, related statistical analyses, and all aspects of manuscript preparation, including drafting, editing, and final content. The study protocol was approved by the local institutional review board at each participating centre and registered at www.ClinicalTrials.gov (NCT01826552).
STUDY PATIENTS
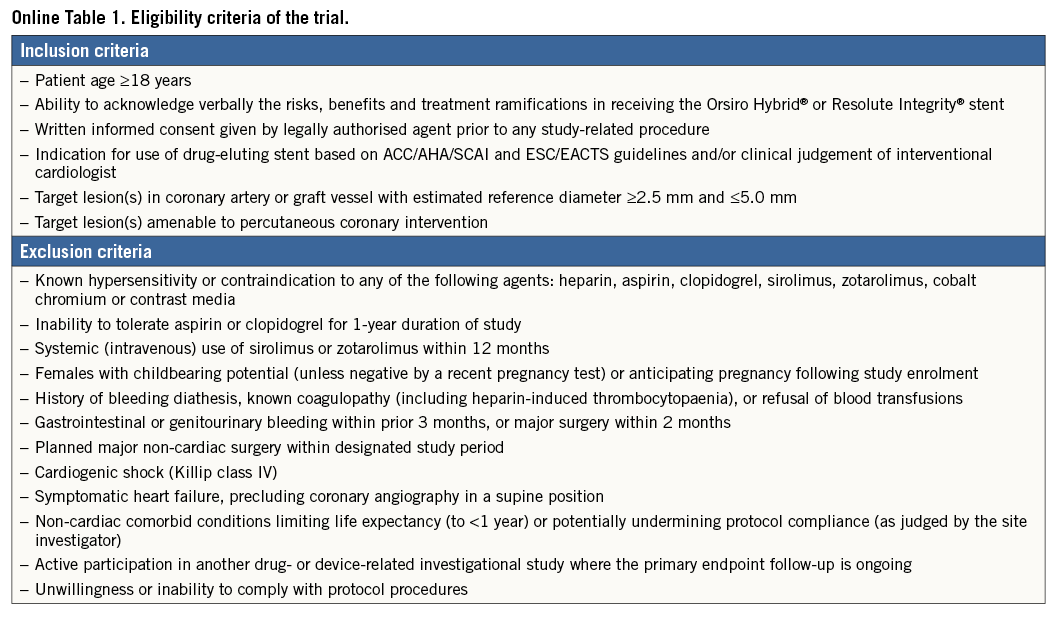
Subjects aged 18 years or older, presenting with symptomatic coronary artery disease and coronary lesions >50%, and indicated for PCI with DES implantation were eligible for enrolment. The decision on the revascularisation modality was based on the current recommendations of the ACC/AHA/SCAI and ESC/EACTS guidelines or the clinical judgement of the interventional cardiologist1,2. Coronary artery disease included stable angina as well as acute coronary syndrome. All participating patients provided written informed consent. Inclusion and exclusion criteria were graded to minimise exclusion of patients, thus reflecting the real-world population at large (Online Table 1).
TREATMENT AND RANDOMISATION
Patients who were planned to undergo PCI after diagnostic angiography were randomly assigned in a 2:1 ratio to either the O-SES or the R-ZES group. Randomisation was carried out via a web-based online randomisation system. The randomisation was stratified by the participating centres. PCI was performed using standard techniques. Dual antiplatelet therapy was recommended for at least 12 months, but was not mandatory. All patients were recommended to undergo angiographic follow-up at nine months post PCI. Clinical follow-up was performed at one, three, nine, and 12 months after the index PCI. Patients were followed up by office visits or telephone contacts.
STUDY ENDPOINTS
The primary endpoint of the trial was in-stent late lumen loss (LLL) at nine months, as measured by quantitative coronary angiography. Secondary angiographic endpoints included in-segment LLL, percentage diameter stenosis, and binary restenosis at nine months. Quantitative analysis of coronary angiographic images (QCA) was performed by specialised technicians who were unaware of the purpose of this study. The analysis was performed at Seoul National University Bundang Hospital Cardiovascular Center. The Cardiovascular Angiography Analysis System 5.9.2 QCA software (Pie Medical Imaging, Maastricht, The Netherlands) was used for automated contour detection and quantification. All QCA measurements of the target lesion were obtained within the stented segment (in-stent), and over the entire segment comprising the stent and its proximal and distal margins (in-segment) up to 5 mm. Secondary clinical endpoints included all-cause and cardiac death, clinically driven target lesion revascularisation (TLR), clinically driven target vessel revascularisation (TVR), myocardial infarction (MI) (target or non-target vessel-related), definite or probable stent thrombosis, and target lesion failure (TLF, a composite of cardiac death, TLR and target vessel-related MI) at 12 months. Clinical events were defined according to the recommendations of the Academic Research Consortium and the Third Universal Definition of MI18,19.
STATISTICAL ANALYSIS
The primary endpoint of nine-month LLL was compared using the Student’s t-test. Assuming a mean LLL of 0.30±0.54 mm for both stents20, we calculated that the enrolment of 375 patients (250 and 125 for the O-SES and R-ZES groups, respectively) would provide a 90% statistical power to confirm the non-inferiority margin of 0.20 mm at a one-sided significance level of 0.05 and an expected dropout rate of 30%21. Sequential superiority testing was performed when the null hypothesis of non-inferiority was rejected. The primary endpoint analysis was performed on the basis of the index lesion, which was determined randomly before the angiographic analysis. Per-lesion and per-treatment analyses were also performed. For the per-lesion analysis, a generalised estimating equations model that used an exchangeable working correlation matrix was used to assess the treatment effect by taking into account the clustering effect within a patient.
All primary and secondary endpoints were analysed on an intention-to-treat basis. Per-treatment analyses were carried out on the primary endpoint, which was intended for descriptive purposes. Secondary clinical endpoints were compared with the Cox proportional hazards model. Kaplan-Meier survival curves were constructed. Binary variables were compared with the use of the χ² test or Fisher’s exact test, and continuous variables were compared with an independent t-test or Wilcoxon’s signed-rank test when appropriate. Exploratory subgroup analysis was performed. Statistical analyses were performed by using R programming, version 3.1.0 (The R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org). A two-sided p-value <0.05 was considered statistically significant.
Results
BASELINE CHARACTERISTICS
Among a total of 372 patients enrolled, 250 were assigned to the O-SES group and 122 to the R-ZES group (Figure 1). Table 1 shows the baseline characteristics of the study population. There were no significant differences in patient characteristics between the assigned groups. The mean age was 65 years, and 71% were male. Sixty-six percent had hypertension, and 26% had diabetes mellitus. The clinical diagnosis was acute coronary syndrome in 47% of the patients, including 9% with ST-segment elevation myocardial infarction.
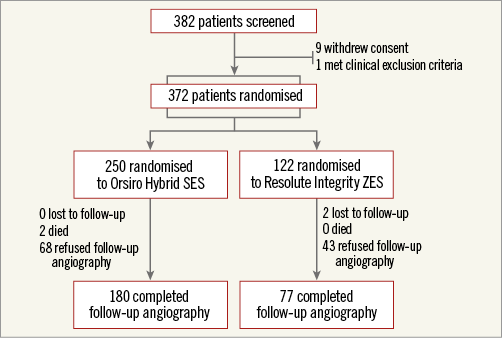
Figure 1. Study flow chart. SES: sirolimus-eluting stent; ZES: zotarolimus-eluting stent
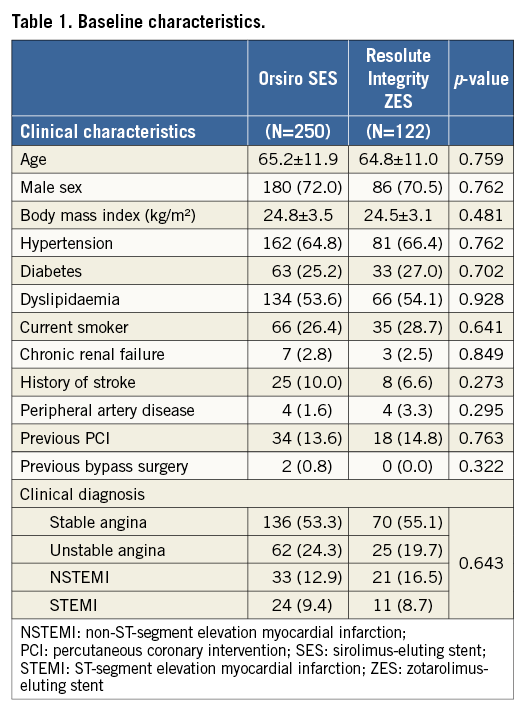
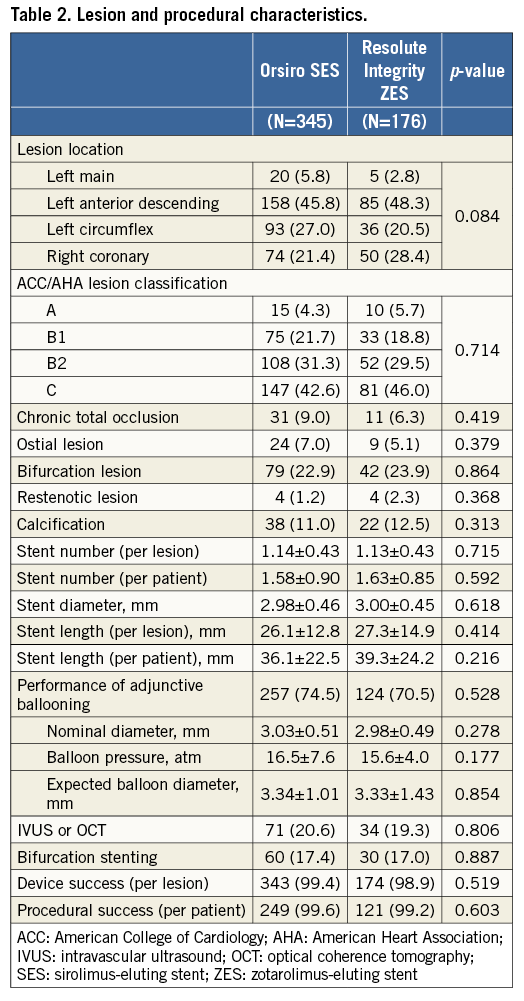
Table 2 shows the data on baseline lesion and procedural characteristics of all treated lesions. Among a total of 521 lesions, the left main coronary artery comprised 5% and the left anterior descending artery 47%. Seventy-four percent of the lesions met the B2/C criteria according to the American College of Cardiology/American Heart Association (ACC/AHA) classification. An adjunctive intracoronary imaging study was carried out in 20%, and bifurcation stenting was required in 17% of the lesions. No significant differences between the groups were present in terms of lesion and procedural factors.
ANGIOGRAPHIC OUTCOMES
Angiographic analyses of the index lesions before and after the index procedure and at nine-month follow-up are shown in Table 3. There were no significant differences before and after the procedures in terms of lesion parameters. Before the procedures, the reference diameter was 2.92 mm, minimal lumen diameter 0.90 mm, and diameter stenosis 74%. Acute gain after PCI was 1.62±0.45 mm, which was similar in both groups.
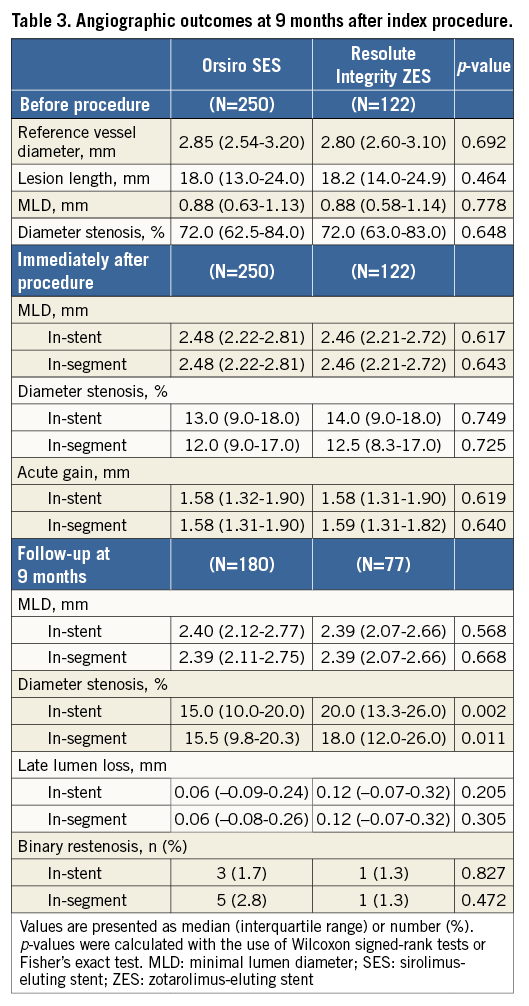
Follow-up angiography was carried out in 69% of the patients after a median of 302 days following the index PCI. The median in-stent LLL, the primary endpoint, was 0.06 mm (interquartile range [IQR], –0.09 to 0.24 mm) and 0.12 mm (IQR, –0.07 to 0.32 mm) in the O-SES and R-ZES groups, respectively. Figure 2A shows the hypothesis testing for the primary endpoint. The upper margin of the difference was within the predefined non-inferiority margin of 0.20 mm (p for non-inferiority <0.001). Superiority testing did not show a statistically significant difference (p for superiority=0.283). In-segment LLL showed similar patterns. Diameter stenosis at nine months post PCI was lower in the O-SES group than in the R-ZES group, significantly for in-stent and marginally for in-segment measurements. The binary restenosis rate was low in both of the groups.
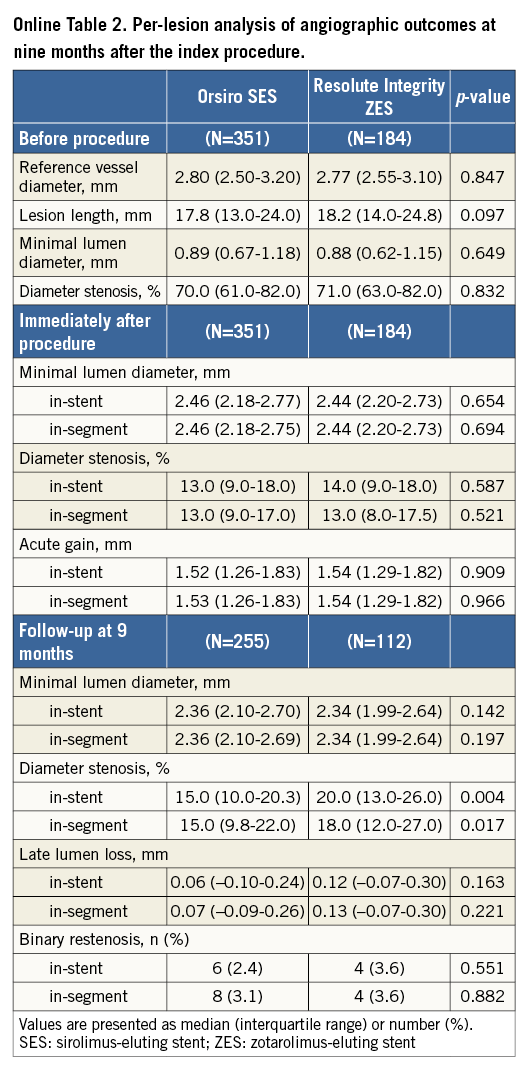
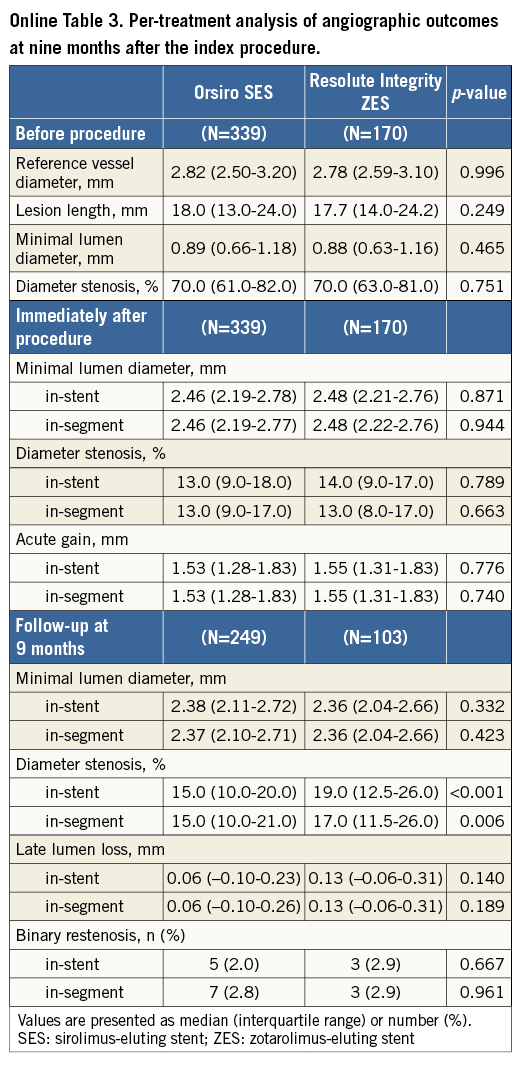
Per-lesion analyses are shown in Online Table 2. In-stent LLL was 0.06 mm (IQR, –0.10 to 0.24 mm) and 0.12 mm (IQR, –0.07 to 0.30 mm) in the O-SES and R-ZES groups, respectively (p=0.163). Online Table 3 shows the per-treatment analyses, in which in-stent LLL was shown to be 0.06 mm (IQR, –0.10 to 0.23 mm) and 0.13 mm (IQR, –0.06 to 0.31 mm) (p=0.140).
CLINICAL OUTCOMES
Table 4 shows a comparison of clinical outcomes of the study groups within 12 months. No significant differences were present in terms of clinical endpoints. As shown in Figure 2B, TLF, a composite of cardiac death, non-fatal MI, and TLF, occurred in 2.4% and 3.3% of the patients in the O-SES and R-ZES groups, respectively (p=0.621). No cases of stent thrombosis were identified.
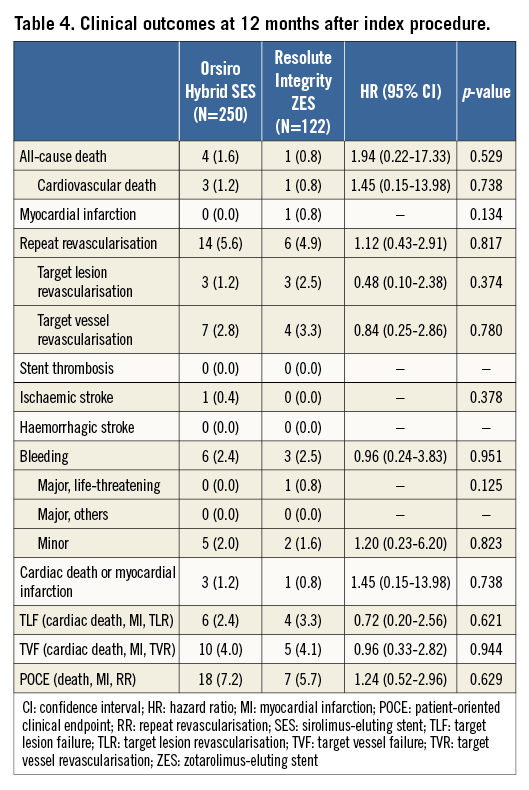
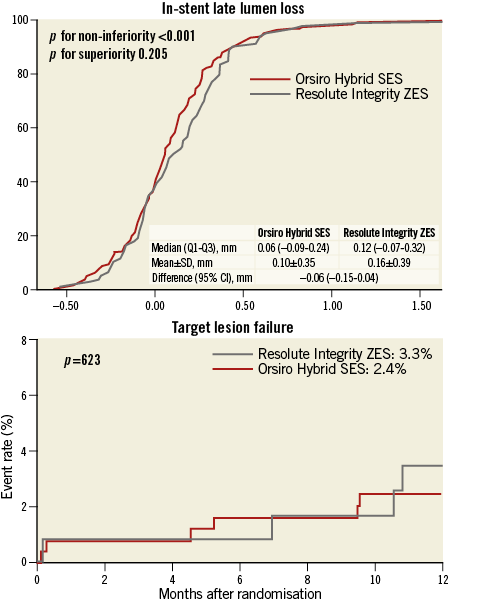
Figure 2. Primary angiographic and secondary clinical endpoint analysis. A) In-stent late lumen loss at nine months. B) Target lesion failure at 12 months after the index procedure. The red line represents the Orsiro biodegradable polymer sirolimus-eluting stent, while the grey line represents the Resolute Integrity durable polymer zotarolimus-eluting stent.
SUBGROUP ANALYSIS
Subgroup analyses for the primary endpoint, in-stent LLL, are shown in Figure 3. The difference in LLL did not vary significantly according to the clinical and angiographic characteristics except for the diabetic subgroup. R-ZES tended to outperform in diabetic patients, while O-SES tended to be better in the non-diabetic subgroup, with a significant interaction (p for interaction=0.033). The median in-stent LLL in the diabetic subgroup was 0.14 mm (IQR, 0.05 to 0.35 mm) and 0.08 (IQR, –0.08 to 0.348 mm) in the O-SES and R-ZES groups, respectively (p=0.169), while it was 0.02 (IQR, –0.11 to 0.21 mm) and 0.13 (–0.05 to 0.31 mm) in the non-diabetic subgroup (p=0.066).
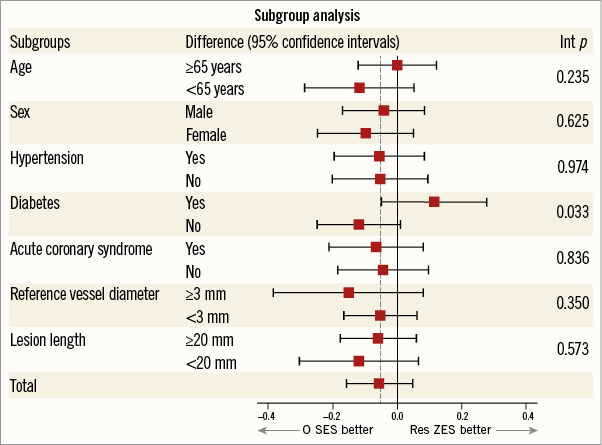
Figure 3. Subgroup analysis. Stratified analyses for several subgroups of the primary endpoint of in-stent late lumen loss. Differences are the mean of the Orsiro biodegradable polymer sirolimus-eluting stent (O-SES) minus Resolute Integrity durable polymer zotarolimus-eluting stent (R-ZES). The horizontal lines represent 95% confidence intervals. Int p: interaction p-values
Discussion
In this study, we showed that O-SES was non-inferior compared to R-ZES in terms of the primary angiographic endpoint, in-stent LLL at nine months. There were no significant differences in clinical outcomes between the two stents. Compared to the R-ZES group, the O-SES group showed a lower percentage of diameter stenosis at nine months.
The findings of this study confirm the good performance of both the O-SES and the R-ZES. R-ZES is one of the most widely used contemporary DES worldwide. The Integrity platform has been utilised in the Resolute Integrity instead of the Driver® bare metal stent platform (Medtronic), which was used in the previous versions. The Integrity stent platform has a 90 μm strut thickness and a 1.12 mm crossing profile. The manufacturing process of the continuous sinusoidal technology promises enhanced flexibility and deliverability, as well as radial and longitudinal strength22. Otherwise, the R-ZES shares the same delivery drug (zotarolimus) and the same BioLinx™ biocompatible polymer (Medtronic) mounted on the same metal alloy (cobalt-chromium) with the previous version, the Endeavor® Resolute ZES (Medtronic). The angiographic and clinical results of the R-ZES group in this study were comparable to the previous outcomes of Endeavor Resolute ZES20,23-27. Until now, two large-scale clinical trials have been published investigating Integrity-platform R-ZES, the DUTCH PEERS and SORT OUT VI trials15,16. The patient characteristics in this study were similar to those seen in the previous trials, except for a lower BMI, a higher rate of diabetes, and a lower frequency of acute coronary syndrome. Adverse clinical event rates were numerically lower in this study.
O-SES represents a newer-generation BP-DES. Several features, such as an ultra-thin 60 μm strut, effective antiproliferative drug (sirolimus), and a hybrid design of passive protection of the metallic surface by a semi-conductive barrier and active drug release from a biodegradable polymer, support the performance as well as the safety of the O-SES. The BIOFLOW-I, a first-in-man trial, showed low in-stent neointimal hyperplasia and low cardiovascular event rates11. The BIOFLOW-II, a randomised controlled clinical trial, proved the non-inferiority of O-SES compared to the XIENCE PRIME® everolimus-eluting stent (X-EES)12. The recently published BIOSCIENCE trial enrolled a large number of patients and randomly assigned them to O-SES or X-EES13. O-SES was shown to be non-inferior to the X-EES, which is considered to be the best among contemporary coronary stents9,28. The rates of clinical adverse events seen in our study are lower than those seen in the previous reports, while neointimal hyperplasia, as assessed by angiography, was similar11,12.
To the best of our knowledge, this is the first study comparing O-SES and R-ZES head to head. In this study, both stents showed good results. While in-stent and in-segment LLL showed no significant difference, percentage diameter stenosis was significantly lower in the O-SES group than in the R-ZES group. The difference became greater in the per-treatment analysis. However, the difference in this angiographic parameter can hardly be translated into an improvement in clinical outcomes. First, it needs to be stated that the percentage of diameter stenosis was not the primary endpoint of this study, but one of the secondary angiographic endpoints. Second, previous larger all-comers trials that were powered to detect the differences in clinical event rates suggest equivalent efficacy of the two devices. The RESOLUTE All-Comers trial actually showed the same event rates between the R-ZES and the X-EES groups24,25. In addition, O-SES showed quite similar outcomes to the X-EES in the BIOSCIENCE trial13. Future studies that are currently underway will provide further insight into the safety and efficacy of the Orsiro SES29.
The significant interaction in the diabetic subgroup shown in this study needs further discussion. Patients with diabetes are at higher risk of adverse events after PCI30. The diabetic milieu attenuates the antirestenoic effects of DES, and the differential effects between different types of DES have attracted attention31,32. In this study, O-SES compared to R-ZES tended to be associated with higher LLL in the diabetic subgroup. However, the BIOFLOW-II trial, in which O-SES and X-EES were compared, found no significant interaction between the stent types and diabetic status12. A pre-specified subgroup analysis of the large-scale BIOSCIENCE trial also showed that the rates of clinical adverse events of O-SES and X-EES were similar in both diabetic and non-diabetic subgroups33. Furthermore, there have been no previous studies that proved differential effects among stents that elute rapamycin analogues according to diabetic status15,16,33. Subgroup analyses in this trial were exploratory and only for hypothesis generation. This finding needs to be tested further in future studies.
Limitations
This study has several limitations. First, this study was designed to detect the non-inferiority margin of the angiographic endpoint. It was underpowered to detect any difference in clinical endpoints. Findings for the secondary endpoints and in the subgroup analyses should be considered to be only of a hypothesis-generating nature. Specifically, this study has limited power for comparison of clinical adverse events. Second, while we tested Resolute Integrity ZES in this study, a newer version of Resolute iterations has been launched on the market, namely the Resolute Onyx™ (Medtronic). However, its design is very similar to that of the Resolute Integrity except for improved visibility. We assume that there is a low probability that the performance of the Onyx version would be vastly different from that of the R-ZES. Third, as the angiographic follow-up was only 69%, a selection bias could have been present. This is an innate drawback for such studies with angiographic endpoints. In addition, the rate of follow-up angiography was balanced between the study groups. Finally, the actual LLL was smaller than expected. Accordingly, from a retrospective viewpoint, our statistical assumption may have been too generous.
Conclusions
O-SES was non-inferior to R-ZES in terms of in-stent LLL at nine months. Angiographic restenosis and clinical adverse event rates were low in both groups. This study confirms the good performance profiles of both of these contemporary coronary stents.
| Impact on daily practice This study showed that the Orsiro Hybrid sirolimus-eluting stent was non-inferior to the Resolute zotarolimus-eluting stent in terms of angiographic outcomes. In addition, the two stents were associated with low angiographic restenosis and clinical adverse events. The results of this study support the safety and efficacy profiles of these two currently available coronary stents. |
Funding
This work was supported by a grant from BIOTRONIK Korea Co., Ltd., Republic of Korea. Besides financial sponsorship, the company had no role in protocol development or the implementation, management, data collection, or analysis of this study.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data