
Abstract
Aims: The ultrathin-strut biodegradable polymer sirolimus-eluting stent (SES) is a new-generation drug-eluting stent (DES) developed to improve the percutaneous treatment of patients with coronary artery disease. Here, we sought to investigate whether the performance of the ultrathin-strut biodegradable polymer SES is superior to that of the benchmark thin-strut fluoropolymer-based everolimus-eluting stent (EES).
Methods and results: We undertook a study-level meta-analysis of trials in which patients receiving percutaneous coronary intervention (PCI) were randomly assigned to either SES or EES. Primary efficacy and safety outcomes were target lesion revascularisation (TLR) and definite/probable stent thrombosis (ST), respectively. Secondary outcomes were myocardial infarction (MI), death, target lesion failure (TLF) and target vessel failure (TVF). A total of 4,853 patients received a PCI with either SES (n=2,816) or EES (n=2,037) in six trials. After a weighted median follow-up of 12 months, patients treated with SES had a risk of TLR (odds ratio [95% confidence interval]: 1.24 [0.83-1.85], p=0.30), definite/probable ST (0.84 [0.53-1.33], p=0.45) and MI related to the target vessel (0.77 [0.55-1.07], p=0.12) comparable to that of patients treated with EES. We found no significant difference with regard to other secondary outcomes.
Conclusions: At one-year follow-up, the ultrathin-strut biodegradable polymer sirolimus-eluting stent displays a performance comparable to that of the fluoropolymer-based everolimus-eluting stent.
Abbreviations
DES: drug-eluting stent
EES: everolimus-eluting stent
MI: myocardial infarction
PCI: percutaneous coronary intervention
SES: sirolimus-eluting stent
ST: stent thrombosis
TLR: target lesion revascularisation
Introduction
In the last decade, metallic drug-eluting stent (DES) platforms have evolved towards percutaneous devices with highly compatible antiproliferative drugs and polymers, supported by thinner backbones1. By virtue of these iterations, percutaneous coronary intervention (PCI) with current-generation metallic DES platforms represents the preferred revascularisation strategy for patients with coronary artery disease (CAD)2.
The thin-strut (81 µm) fluoropolymer-based everolimus-eluting stent (EES) (XIENCE; Abbott Vascular, Santa Clara, CA, USA) is regarded as the benchmark contemporary DES. Indeed, this platform has demonstrated favourable vascular response3 and superior clinical performance in direct and indirect comparisons against various drug-eluting and bare metal stents4. Notwithstanding this, recent refinements in DES technology have led to innovative coatings and metallic scaffold designs, which have allowed a further reduction of strut thickness while retaining biocompatibility and radial strength5. The ultrathin-strut biodegradable polymer sirolimus-eluting stent (SES) (Orsiro; Biotronik, Bülach, Switzerland) has the thinnest struts (60 µm) among contemporary drug-eluting stents6. Despite encouraging preclinical data3, a number of recent randomised clinical trials did not consistently display that the performance of SES is superior to that of fluoropolymer-based EES7-9.
Against this background, we performed a study-level meta-analysis to investigate whether the efficacy and safety of the ultrathin-strut biodegradable polymer SES is superior to that of the thin-strut fluoropolymer-based EES.
Methods
SEARCH STRATEGY AND SELECTION CRITERIA
The details of search strategy and selection criteria are presented in Supplementary Appendix 1 and Supplementary Appendix 2.
DATA COLLECTION AND ASSESSMENT OF RISK OF BIAS
Two investigators (S. Cassese and N. Mankerious) independently assessed publications for eligibility at title and/or abstract level, with divergences resolved by a third investigator (M. Joner). Studies that met inclusion criteria were selected for further analysis. Freedom from bias was independently evaluated for each study by the same investigators in accordance with The Cochrane Collaboration method10. Composite quality scores were not assigned11.
OUTCOME VARIABLES
For this report, the primary efficacy and safety outcomes were target lesion revascularisation (TLR) and definite/probable stent thrombosis (ST), respectively. The main secondary outcome was myocardial infarction (MI). Other secondary outcomes of interest were death, target lesion failure (TLF), the device-oriented composite endpoint including cardiac death, target vessel MI, or ischaemia-driven target lesion revascularisation (ID-TLR), and target vessel failure (TVF), the patient-oriented composite endpoint including cardiac death, target vessel MI, or ischaemia-driven target vessel revascularisation. All endpoints were evaluated in the intention-to-treat population up to 12-month follow-up and according to definitions reported in the original protocols. In case of missing data relevant to the study research question, the principal investigators (for investigator-initiated trials) or the manufacturer (for industry-initiated trials) were contacted directly.
STATISTICAL ANALYSIS
Odds ratio (OR) and 95% confidence intervals (95% CI) were used to compare the outcomes of interest of SES versus EES and pooled using the Mantel-Haenszel fixed-effect model and the DerSimonian and Laird random-effect model. Treatment effect was not assessed in trials in which no events were reported within groups. Heterogeneity between trials was quantified using the I2 statistic accompanied by a chi2 test: I2 values around 25%, 50% and 75% were suggested to indicate low, moderate or high heterogeneity, respectively10. In addition, we estimated the between-study variance (τ2). The possibility of small study effects resulting from publication bias or other biases was examined for primary outcomes by means of visual inspection of funnel plots of the ORs of individual trials against their standard errors. An influence analysis, in which meta-analysis estimates are computed omitting one study at a time, was performed for primary outcomes. We used a chi2 test for subgroup by treatment interaction for two purposes: as first, to investigate the possible time dependence of the risk of definite/probable and definite ST (early –up to 30 days– versus late –beyond 30 days to 12 months) associated with either SES or EES; as second, to test whether the inclusion of patients with acute MI (yes versus no), the protocol-mandated angiographic surveillance (yes versus no), and the type of sponsorship (industry- versus investigator-initiated) were associated with estimated ORs of primary and main secondary outcomes. By using random-effects meta-regression analysis, we assessed the influence on primary and main secondary outcomes of relevant patient characteristics at baseline (proportion of males, diabetics and acute coronary syndromes). Finally, we determined the power of our random-effects meta-analysis to detect a pre-specified 30% relative risk reduction (RRR) of primary and secondary outcomes with SES conditional on the observed precision of the pooled estimate12. The 30% RRR threshold was chosen because it approximates the nominal effect size seen in cardiovascular trials that is both clinically meaningful and realistic. This study was reported in compliance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement (Supplementary Table 1)13. All analyses were performed in R, version 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria). This study is registered with PROSPERO, number CRD42017078538.
Results
ELIGIBLE STUDIES
Figure 1 shows the flow diagram for the trial selection process. Finally, we selected six trials (five reported with full-length manuscripts7-9,14,15 and one reported with a meeting presentation [Saito S., Japan Circulation Society Congress, 2017, Kanazawa, Japan]) in which a total of 4,853 PCI patients were randomly assigned to either SES (n=2,816) or EES (n=2,037). For two out of six trials, the investigators15 and the manufacturer (Saito S., Japan Circulation Society Congress, 2017, Kanazawa, Japan) agreed to provide unpublished study-level data concerning clinical and angiographic features, as well as clinical outcomes. The main characteristics of the trials included are described in detail in Supplementary Table 2. Briefly, patients with obstructive chronic/stable or unstable CAD were randomised to PCI with SES versus EES. Patients assigned to EES received the XIENCE V®/XIENCE Prime®/XIENCE Xpedition® (Abbott Vascular) stent. All but one trial15 had a multicentre design. Two7,8 out of six trials enrolled patients with acute MI. Three studies8,14,15 required per protocol a control angiography six to nine months after index PCI: in these trials the percentage of patients with angiographic follow-up data ranged from 74.3% to 85.5%. At the time of index PCI, all patients received loading doses of thienopyridines, as well as aspirin. In all cases, a maintenance dose of aspirin was recommended indefinitely, whilst the length and the type of thienopyridine prescription depended on clinical indication. The adherence to prescribed dual antiplatelet therapy was available for 4,364 patients after 12 months: at this time point 2,067 (80.8%) of 2,558 patients treated with SES and 1,387 (76.7%) of 1,806 patients treated with EES were actually on dual antiplatelet therapy. All interventions were performed in accordance with standard of care including anticoagulation, stent deployment optimisation or use of intravascular imaging techniques, at the operators’ discretion or according to protocols. In three trials9,14,15 the primary endpoint consisted of angiographic (namely, in-stent late lumen loss) or imaging (namely, neointimal tissue coverage) measures of efficacy. The remaining trials were powered for composite clinical endpoints. Two7,14 out of six trials had outcome data beyond 12 months available; however, to ensure a homogeneous observation period across included trials the current report analysed adverse events occurring up to 12 months after index PCI. The definitions of outcomes are reported in Supplementary Table 3 and the risk of bias among studies is reported in Supplementary Table 4.
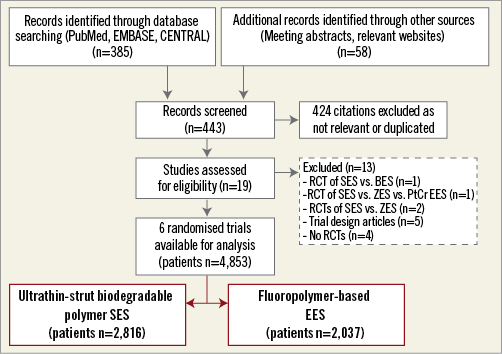
Figure 1. PRISMA flow chart for the trial selection process. BES: biolimus-eluting stent; EES: fluoropolymer-based everolimus-eluting stent; PRISMA: Preferred Reporting Items for Systematic reviews and Meta-Analyses; PtCr EES: platinum-chromium biodegradable polymer everolimus-eluting stent; RCT(s): randomised controlled trial(s); SES: ultrathin-strut biodegradable polymer sirolimus-eluting stent; ZES: zotarolimus-eluting stent
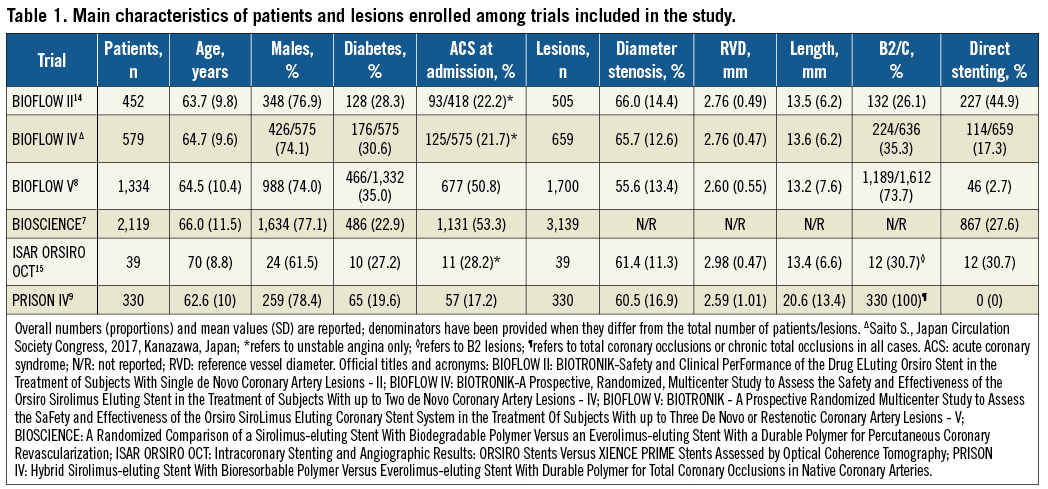
The baseline characteristics of individuals enrolled are shown in Table 1. Patients were more often male, with a median age of 64.6 years (interquartile range, 63.7-66.0) and roughly one third were diabetics. Nearly a fourth of participants presented with ACS at the time of index PCI. Overall, at baseline angiography, treated lesions presented a mean reference vessel diameter of 2.73 mm, a diameter stenosis of 61.6%, and a length of 14.9 mm, and more than half had a complex morphology. Among those randomised, 4,756 patients (98.0%) were available for assessment of outcomes of interest after a weighted median follow-up of 12 months (mean 11.0±2.5).
CLINICAL OUTCOMES
Figure 2 displays a summary of risk estimates as well as the power of the random-effects meta-analysis to detect a 30% RRR of primary and secondary outcomes associated with SES. The details of risk estimates for each study endpoint are presented in Supplementary Figure 1-Supplementary Figure 3.
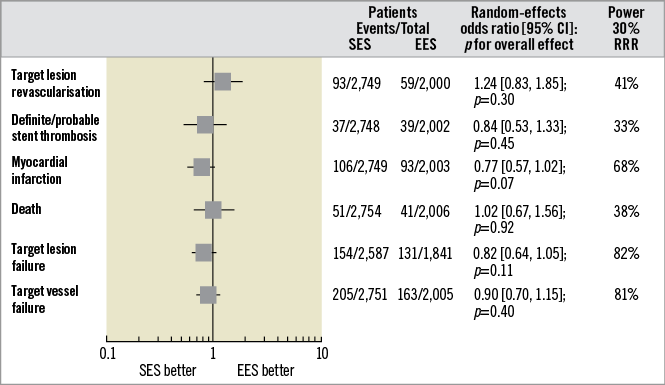
Figure 2. Summary of risk estimates for primary and secondary outcomes for SES versus EES. Plot of random-effects odds ratios associated with SES versus EES. The diamonds indicate the point estimate and the left and the right ends of the lines the 95% confidence intervals (CI). The power of random-effects meta-analysis to detect a pre-specified 30% RRR of primary and secondary outcomes with SES conditional on the observed precision of the pooled estimate is shown in the last column on the right. EES: fluoropolymer-based everolimus-eluting stent; RRR: relative risk reduction; SES: ultrathin-strut biodegradable-polymer sirolimus-eluting stent
The primary efficacy outcome of TLR occurred in 152 patients (3.2%) (Supplementary Figure 1A). Patients treated with SES had a risk of TLR comparable to that observed with EES (3.4% versus 2.9%; OR [95% CI] 1.24 [0.83-1.85], p=0.30; I2=15%). ID-TLR occurred in 131 patients (2.7%). Patients treated with SES had a risk of ID-TLR comparable to that observed with EES (3.0% versus 2.4%; 1.27 [0.83-1.95], p=0.26; I2=15%). Of interest, in those trials9,14,15 with protocol-mandated control angiography, the degree of in-stent late lumen loss after a median of nine months [mean 8.0±1.7] was comparable between SES and EES (weighted mean difference [95% CI] –0.00 [–0.06, 0.06], p=0.99; I2=0%; data available for 695 patients).
The primary safety outcome of definite/probable ST occurred in 76 patients (1.6%) (Supplementary Figure 1B). Patients treated with SES had a risk of definite/probable ST comparable to that observed with EES (1.3% versus 1.9%; 0.84 [0.53-1.33], p=0.45; I2=0%). The risk of early (0.90 [0.50-1.61], p=0.72; I2=0%) and late (0.73 [0.33-1.61], p=0.43; I2=0%) definite/probable ST was comparable between SES and EES (data available for 4,423 participants). There was no time-dependent risk of definite/probable ST associated with SES versus EES (p for interaction–pint=0.67). Definite ST occurred in 25 patients (0.5%). Patients treated with SES had a risk of definite ST comparable to that observed with EES (0.6% versus 0.4%; 1.48 [0.64-3.44], p=0.36; I2=0%). The risk of early (1.75 [0.49-6.25], p=0.39; I2=0%) and late (1.03 [0.09-11.34], p=0.98; I2=64%) definite ST was comparable between SES and EES (data available for 4,421 participants). There was no time-dependent risk of definite ST associated with SES versus EES (pint=0.70).
The main secondary outcome of MI occurred in 199 patients (4.2%) (Supplementary Figure 2). Patients treated with SES displayed a tendency to lower risk of MI as compared to EES (3.8% versus 4.6%; 0.77 [0.57-1.02], p=0.07; I2=0%). MI related to the target vessel occurred in 166 patients (3.7%, data available for 4,423 participants). Patients treated with SES had a risk of MI related to the target vessel comparable to EES (3.5% versus 4.1%; 0.77 [0.55-1.07], p=0.12; I2=11%).
Death occurred in 92 patients (1.9%, data available for 4,760 individuals). Patients treated with SES had a risk of death similar to that observed with EES (1.8% versus 2.0%; 1.02 [0.67-1.56], p=0.92; I2=0%). Cardiac death occurred in 53 patients (1.1%) (Supplementary Figure 3A). Patients treated with SES displayed a risk of cardiac death comparable to that observed with EES (0.9% versus 1.4%; 0.76 [0.44-1.31], p=0.32; I2=0%).
TLF occurred in 285 patients (6.4%, data available for 4,428 participants) (Supplementary Figure 3B). Patients treated with SES had a risk of TLF comparable to that of EES (5.9% versus 7.1%; 0.82 [0.64-1.05], p=0.11; I2=0%).
TVF occurred in 368 patients (7.7%) (Supplementary Figure 3C). Patients treated with SES displayed a risk of TVF comparable to that of EES (7.4% versus 8.1%; 0.90 [0.70-1.15], p=0.40; I2=18%).
SMALL STUDY EFFECTS, INFLUENCE AND SENSITIVITY ANALYSES
By visual inspection, funnel plots for primary efficacy and safety outcomes revealed no evidence for small study effects (Supplementary Figure 4A, Supplementary Figure 5A). The influence analysis demonstrated that no single study significantly altered the direction of the summary ORs for primary outcomes (Supplementary Figure 4B, Supplementary Figure 5B). The subgroup by treatment interaction analysis for primary and main secondary outcomes is reported in Figure 3: the inclusion of patients with acute MI (pint≥0.45), the angiographic surveillance (pint≥0.82) and the sponsorship (pint≥0.14) did not affect the risk estimates for TLR and definite/probable ST. The risk estimate for MI was independent from angiographic surveillance (pint=0.49) and sponsorship (pint=0.52). In contrast, SES was associated with a lower risk of MI as compared to EES across trials enrolling patients with acute MI (0.71 [0.52-0.98], p=0.039, pint=0.042). The meta-regression analysis found that the treatment effect regarding TLR (p=0.47, 0.11 and 0.49), definite/probable ST (p=0.88, 0.99 and 0.49) and MI (p=0.20, 0.13 and 0.36) was not significantly affected by the baseline proportion of males, diabetics and acute coronary syndromes, respectively.
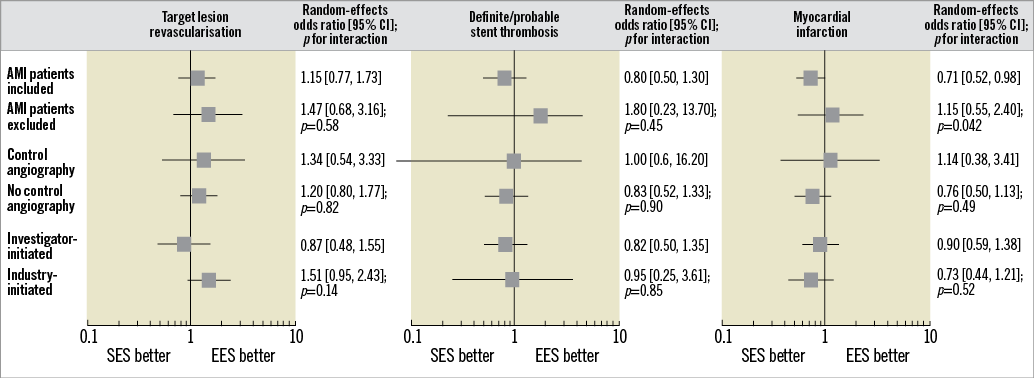
Figure 3. Summary of risk estimates for primary and main secondary outcomes for SES versus EES. Plot of random-effects odds ratios associated with SES versus EES according to subgroups of interest. The diamonds indicate the point estimate and the left and the right ends of the lines the 95% confidence intervals (CI); p-values for interaction are from chi2 test. AMI: acute myocardial infarction; EES: fluoropolymer-based everolimus-eluting stent; SES: ultrathin-strut biodegradable polymer sirolimus-eluting stent
Discussion
This meta-analysis investigated the outcomes of PCI patients treated with either ultrathin-strut biodegradable polymer SES or thin-strut fluoropolymer-based EES. At one-year follow-up as compared to EES, SES showed similar overall efficacy and safety. The tendency towards lower risk of MI associated with SES versus EES and the modification of treatment effect with respect to this outcome in trials enrolling patients with acute MI require further investigation.
The ultrathin-strut, biodegradable polymer SES investigated in the present study consists of a cobalt-chromium scaffold (strut thickness 60 µm for stent diameters up to 3.0 mm and 80 µm for stent diameters >3.0 mm) coated with a fully biodegradable dual polymer. This coating is composed of a thin layer of amorphous, hydrogen-rich, silicon carbide, which is in contact with the stent surface, and an asymmetric circumferential layer containing a matrix of poly-L-lactic acid (PLLA) loaded with the antiproliferative drug sirolimus (1.4 μg/mm2 of stent surface). Of note, the PLLA has the slowest degradation kinetic among biodegradable polymers, enabling a more controlled release of antiproliferative drug from the stent surface6. In this meta-analysis we investigated the aggregate data from six randomised trials with ≈5,000 PCI patients allocated to either ultrathin-strut, biodegradable polymer SES or the best-in-class thin-strut fluoropolymer-based EES. In contrast with other meta-analyses4,16,17, we analysed the totality of aggregate data from randomised trials comparing SES versus EES, by including unpublished data relevant to the study research question.
Firstly, in this meta-analysis patients treated with SES showed a risk of TLR at one-year follow-up comparable to that of patients assigned to EES. This result lends support to the comparable angiographic performance of SES and EES observed in our analysis and confirms the favourable efficacy profile of SES observed in broadly inclusive patient populations18. However, the data accumulated have little statistical power to rule out any clinically relevant benefit in terms of TLR associated with SES.
Secondly, we found comparable risk of ST after PCI with SES versus EES without time dependence of risk estimation. This study confirms once more the excellent safety profile of contemporary high-performance DES, with an overall rate of definite ST at 12 months of 0.5% across nearly 5,000 patients, including those presenting with acute MI and highly complex coronary anatomies (such as chronic total occlusions). However, the available sample size remains insufficient to address a measurable advantage of SES versus EES with respect to this endpoint. In addition, despite the fact that longer follow-up is instrumental in unravelling whether SES are safer than EES after complete degradation of the polymer, available outcome data beyond 12 months do not support a lower risk of thrombotic events in patient cohorts receiving a PCI therapy with SES against EES19.
Thirdly, this analysis refutes the recent hypothesis that the ultrathin-strut biodegradable polymer SES lowers the risk of TLF by virtue of fewer MI events as compared to EES8. Indeed, despite the fact that in our analysis the risk of MI trended lower with SES as compared to EES, the risk of MI related to the target vessel and TLF was comparable between treatment groups. Preclinical data found both DES platforms under investigation to be associated with low thrombogenicity and accelerated endothelialisation owing to their thin-strut design and biocompatible carriers3,20,21. Although the coating formulation of SES aims to reduce further the interaction of metal components with the vessel wall6, a potential lower risk of MI with SES as compared to EES has yet to be demonstrated. Moreover, the lack of complete details regarding the types of MI event (periprocedural versus spontaneous) and the exploratory nature of subgroup analyses performed in this report prevent full disclosure of the variation of treatment effect for this outcome in patients with acute MI, for which we observed a significant interaction. For this reason, the proof of superior performance of SES versus EES in patients with acute MI remains to be studied22.
Study limitations
A number of limitations inherent to the present study should be acknowledged. First, this meta-analysis was based on aggregate data: an individual patient-data meta-analysis remains necessary to investigate a variation of treatment effect across different subgroups of patients, such as those presenting with acute MI. Second, the median follow-up of one year prevents investigating whether SES performs better than EES beyond the time of complete resorption of the polymer. Third, this study has insufficient statistical power to investigate the comparative efficacy of SES and EES with respect to outcomes other than MI, TLF and TVF. Fourth, the protocol-mandated surveillance angiography in three trials9,14,15 may have magnified differences in the absolute proportion of revascularisations across groups, though we found no interaction between treatment effect and control angiography. Finally, the assessment of publication bias was based on a limited number of trials, limiting the performance of this assessment.
Conclusions
At one-year follow-up, clinical outcomes of PCI patients treated with an ultrathin-strut biodegradable polymer sirolimus-eluting stent were broadly comparable to those of patients treated with a thin-strut fluoropolymer-based everolimus-eluting stent.
| Impact on daily practice The ultrathin-strut biodegradable polymer sirolimus-eluting stent is a new-generation drug-eluting stent developed to improve the percutaneous treatment of patients with coronary artery disease. In this meta-analysis of aggregate data from six randomised trials with circa 5,000 patients the ultrathin-strut biodegradable polymer sirolimus-eluting stent displayed a performance broadly comparable to that of the benchmark fluoropolymer-based everolimus-eluting stent at one-year follow-up. Any potential benefit associated with the ultrathin-strut biodegradable polymer sirolimus-eluting stent should be explored in more complex cohorts of patients and with a clinical follow-up beyond one year. |
Guest Editors
This paper was guest edited by Antonio Colombo, MD, FACC, FESC, FSCAI; Department of Interventional Cardiology, EMO GVM Centro Cuore Columbus, Milan, Italy, and Alec Vahanian, MD, PhD; Department of Cardiology, Hôpital Bichat-Claude Bernard, and University Paris VII, Paris, France.
Conflict of interest statement
R.A. Byrne reports receiving lecture fees from B. Braun Melsungen AG, Biotronik and Boston Scientific, and research grants to the institution from Boston Scientific and HeartFlow, outside the submitted work. N. Mankerious is the recipient of a research fellowship grant funded by the European Association of Percutaneous Cardiovascular Interventions (EAPCI). A. Kastrati reports holding patents related to drug-eluting stent technology, outside the submitted work. M. Joner reports receiving personal fees from OrbusNeich, grants and personal fees from Biotronik, personal fees from coramaze, personal fees from AstraZeneca, and personal fees from Bristol-Myers-Squibb, outside the submitted work. The other authors have no conflicts of interest to declare. The Guest Editor A. Vahanian is a consultant for Edwards Lifesciences. The Guest Editor A. Colombo has no conflicts of interest to declare.
Supplementary data
Supplementary Appendix 1. Search strategy and selection criteria.
Supplementary Appendix 2. Search strategy: PubMed.
Supplementary Table 1. PRISMA checklist.
Supplementary Table 2. Main characteristics of trials included in the study.
Supplementary Table 3. Definitions of clinical outcomes according to protocols of trials included in the study.
Supplementary Table 4. Assessment of risk of bias.
Supplementary Figure 1. Risk estimates of primary outcomes for SES versus EES.
Supplementary Figure 2. Risk estimates of main secondary outcome for SES versus EES.
Supplementary Figure 3. Risk estimates of other secondary outcomes for SES versus EES.
Supplementary Figure 4. Funnel plot and influence analysis for target lesion revascularisation.
Supplementary Figure 5. Funnel plot and influence analysis for definite/probable stent thrombosis.
To read the full content of this article, please download the PDF.

