
Abstract
Aims: The durable polymer platinum-chromium everolimus-eluting stent (PtCr-EES) is a new-generation drug-eluting stent (DES) with a platinum-enriched metallic platform developed to improve the percutaneous treatment of patients with coronary artery disease. We sought to investigate the performance of durable polymer PtCr-EES versus other new-generation DES.
Methods and results: We undertook a meta-analysis of trials in which patients receiving percutaneous coronary intervention (PCI) were randomly assigned to durable polymer PtCr-EES versus other new-generation DES (other DES). Primary efficacy and safety outcomes were target lesion revascularisation (TLR) and definite/probable stent thrombosis (ST), respectively. Secondary outcomes were myocardial infarction (MI), target vessel revascularisation (TVR), death, cardiac death and longitudinal stent deformation (LSD). A total of 11,036 patients in seven trials received a PCI with either durable polymer PtCr-EES (n=6,613) or other DES (n=4,423). This latter group comprised patients treated with biolimus- (n=325), cobalt-chromium everolimus- (n=1,940) or zotarolimus-eluting stents (n=2,158). After a median follow-up of 12 months (interquartile range 12-24), durable polymer PtCr-EES displayed a risk of TLR (odds ratio 0.98, 95% confidence interval [CI]: 0.75-1.29; p=0.90) and definite/probable ST (0.89 [0.55-1.45]; p=0.63) comparable to that of other DES. However, the durable polymer PtCr-EES was associated with a higher risk of LSD (12.05 [1.60-90.71], p=0.02) compared to other DES. There was no significant difference with regard to other secondary outcomes nor was there heterogeneity across trials.
Conclusions: At one-year follow-up, the durable polymer PtCr-EES displays a performance comparable to that of other new-generation DES platforms.
Abbreviations
DES: drug-eluting stent
EES: everolimus-eluting stent
LSD: longitudinal stent deformation
PCI: percutaneous coronary intervention
PtCr: platinum-chromium
ST: stent thrombosis
TLR: target lesion revascularisation
Introduction
Contemporary high-performance drug-eluting stent (DES) platforms are regarded as a first-line therapy for a large spectrum of coronary artery disease (CAD) patients, even for those individuals presenting with complex clinical and angiographic characteristics1. This achievement results from the continuous iterative process to which active drugs, polymer coatings and supportive metallic backbones have been subject in recent years2.
Despite drugs and polymers having attracted considerable interest, the role of underlying scaffolds in DES-treated patients remains less studied3,4. On the one hand, the cobalt-chromium (CoCr) alloy, used in the majority of contemporary DES platforms, has enabled a reduction in stent strut thickness and improved device deliverability, though at the expense of greater recoil as compared with stainless steel alloy5. On the other hand, the introduction of platinum-enriched platforms and innovative backbone designs has permitted a further reduction of strut thickness while improving radiopacity and retaining radial strength at the cost of inferior longitudinal stent stability5. Preliminary investigations of stent platforms based on platinum-chromium (PtCr) alloy have demonstrated less thrombogenicity, favourable endothelial surface coverage6 and better conformability to the vessel wall after implantation7. Whether these properties translate into measurable clinical benefits is still open to question.
The durable polymer, PtCr-based, everolimus-eluting stent (PtCr-EES) (PROMUS Element™; Boston Scientific, Marlborough, MA, USA) has been studied against other new-generation stainless steel or CoCr-DES platforms in a number of randomised clinical trials8-14. However, the overwhelming majority of these trials had a non-inferiority design and sufficient statistical power only for composite or surrogate outcomes.
Against this background, the present meta-analysis investigates the outcomes of patients treated with durable polymer PtCr-EES as compared with other new-generation DES.
Methods
SEARCH STRATEGY AND SELECTION CRITERIA
All details of the search strategy and selection criteria are provided in the Online Appendix.
DATA COLLECTION AND ASSESSMENT OF RISK OF BIAS
Two investigators (S. Cassese and G. Ndrepepa) independently assessed publications for eligibility at title and/or abstract level, with divergences resolved by a third investigator (M. Fusaro). Studies that met inclusion criteria were selected for further analysis. Freedom from bias was evaluated for each study by the same investigators, in accordance with the Cochrane Collaboration method15. No formal quality score adjudication was performed16.
OUTCOME VARIABLES
For this report, the primary efficacy and safety outcomes were target lesion revascularisation (TLR) and definite/probable stent thrombosis (ST), respectively. Secondary outcomes of interest were myocardial infarction (MI), target vessel revascularisation (TVR), death, cardiac death and longitudinal stent deformation (LSD). All endpoints were evaluated at the longest available follow-up according to the definitions of the original protocols.
STATISTICAL ANALYSIS
The odds ratio (OR) and 95% confidence interval (95% CI) were used as summary statistics and were derived for comparison of durable polymer PtCr-EES versus other new-generation DES (other DES). The Mantel-Haenszel random effects model (DerSimonian and Laird) was used to calculate pooled ORs. Treatment effect was not assessed in the trials in which no events were reported within groups. The Breslow-Day chi2 test and the I2 statistic were used to test heterogeneity across the studies: I2 values of <25%, 25-50% or >50% indicated low, moderate or high heterogeneity15. The restricted maximum likelihood method (Tau2) took into account the occurrence of residual heterogeneity.
For the primary outcomes we performed: (i) a visual estimation of funnel plots, as well as statistical tests to evaluate the possibility of publication bias17-19; (ii) an influence analysis, in which meta-analysis estimates are computed omitting one study at a time; (iii) a trial sequential analysis, in which meta-analysis sample size calculations are combined with the threshold of statistical significance20. A sensitivity analysis evaluated the extent to which the comparator DES, the polymer serving as drug carrier (durable/bioresorbable) or the strut thickness (≥81 µm) in the control DES group, the all-comers design of the trials or the protocol-mandated control angiography might have influenced the risk calculations for the primary outcomes.
Statistical analysis was performed using Review Manager (RevMan), Version 5.3 (The Cochrane Collaboration, Copenhagen, Denmark), Stata 11.4 (StataCorp, College Station, TX, USA) and trial sequential analysis (TSA), version 0.9 Beta software packages. This study was registered with PROSPERO, number CRD42016038594 and conducted in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement21.
Results
ELIGIBLE STUDIES
Figure 1 shows the flow diagram for the trial selection process. Among studies selected for further analyses, four randomised trials were excluded7,22-24: the EVOLVE22 and EVOLVE II23 trials compared two PtCr-EES platforms; the PLATINUM China trial24 compared the durable polymer PtCr-EES with early-generation DES; and the trial of Kim and co-workers7 had a follow-up length <6 months. Finally, we selected seven trials (all with full-length manuscripts8-14) in which a total of 11,036 PCI patients were randomly assigned to durable polymer PtCr-EES (n=6,613) or other DES (n=4,423). Patients assigned to the control group received a biolimus-eluting stent (BES) (Nobori® [Terumo Corp., Tokyo, Japan], or BioMatrix Flex™ [Biosensors Inc., Newport Beach, CA, USA], n=325) in two trials9,11, a cobalt-chromium EES (CoCr-EES) (PROMUS™ [Boston Scientific], or XIENCE V®/XIENCE Prime™ [Abbott Vascular, Santa Clara, CA, USA], n=1,940) in three trials12-14, or a zotarolimus-eluting stent (ZES) (Resolute™/Resolute Integrity® [Medtronic, Santa Rosa, CA, USA], n=2,158) in two trials8,10.
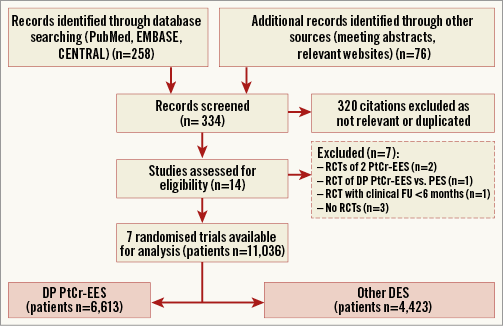
Figure 1. PRISMA flow chart for the trial selection process. DES: drug-eluting stent; DP PtCr-EES: durable polymer platinum-chromium everolimus-eluting stent; FU: follow-up; PES: paclitaxel-eluting stent; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCTs: randomised controlled trials
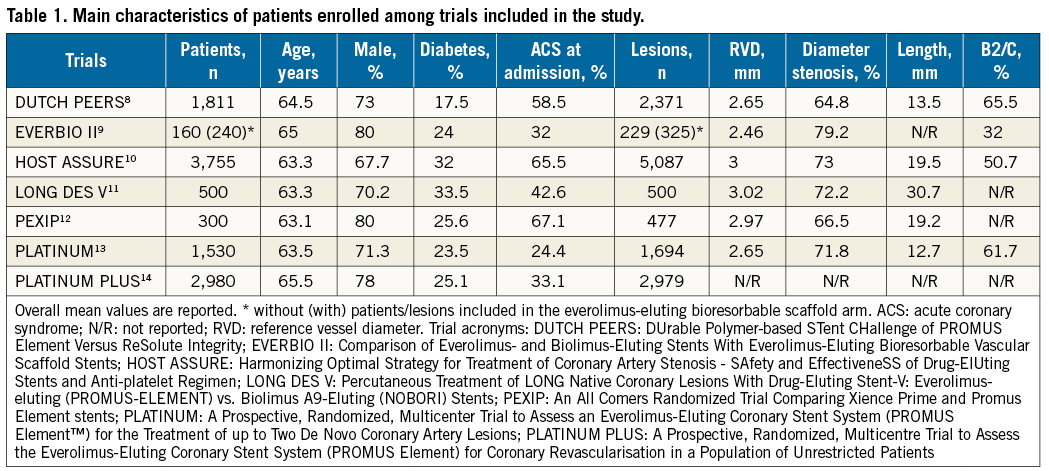
The main characteristics of the trials included are described in detail in Online Table 1. Briefly, patients with obstructive chronic/stable or unstable CAD were randomised to PCI with durable polymer PtCr-EES versus other DES. One trial9 comprised a third treatment arm of patients randomised to an everolimus-eluting bioresorbable vascular scaffold (Absorb; Abbott Vascular); data belonging to this treatment arm were excluded as irrelevant to the study research question. All but two trials9,12 had a multicentre design. Two studies9,11 among those included required a protocol-mandated control angiography nine months after the index procedure; in these trials the percentage of patients with invasive surveillance data ranged from 64.3% to 90.8%. Loading doses of thienopyridines, as well as aspirin, were administered to all patients at the time of index PCI. In all cases, aspirin was recommended indefinitely, whilst the length and the type of thienopyridine prescription depended on clinical indication. All interventions were performed in accordance with standard of care including anticoagulation, stent deployment optimisation or use of intravascular imaging techniques, at the operators’ discretion or according to protocols. In two trials9,11, the primary endpoint consisted of angiographic measures of efficacy (namely, in-stent late lumen loss). For two trials25,26, clinical endpoints after one-year follow-up were available: these data were used for the present report. The definitions of outcomes are reported in Online Table 2 and the risk of bias among studies is reported in Online Table 3.
Baseline characteristics of the individuals enrolled are summarised in Table 1. Patients were mainly men, with a median age of 63.5 years (interquartile range, 63.3-65.0) and one quarter had diabetes. Nearly 45% of participants presented with ACS at the time of enrolment. Overall, the mean reference vessel diameter was 2.80 mm, the baseline diameter stenosis was 71.3%, the length of lesions treated was 19.2 mm, and roughly 60% of lesions had a complex morphology. Among those randomised, 10,810 patients (98.0%) were available for outcome assessment. Median follow-up was 12 months (12-24; mean 17.6±9.5).
CLINICAL OUTCOMES
Figure 2 displays a summary of risk estimates for the primary and main secondary outcomes of this report. The details of risk estimates for each study endpoint are presented in the Online Appendix
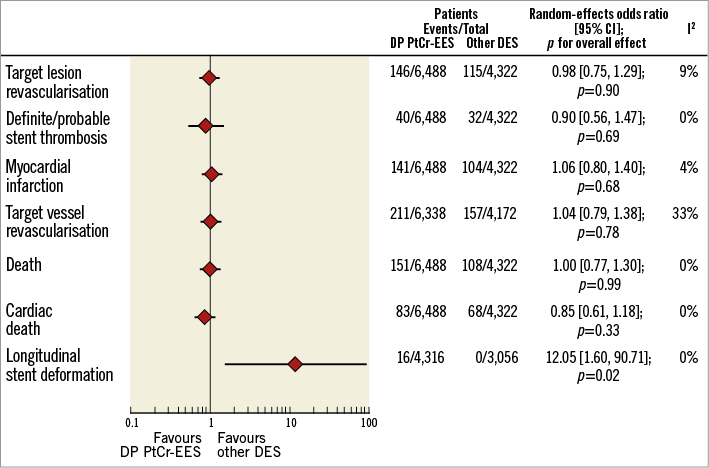
Figure 2. Summary of risk estimates for primary and main secondary outcomes for DP PtCr-EES versus other DES. Plot of odds ratio associated with DP PtCr-EES versus other DES. The diamonds indicate the point estimate and the left and the right ends of the lines the 95% confidence interval (CI). DES: drug-eluting stent; DP PtCr-EES: durable polymer platinum-chromium everolimus-eluting stent
TLR (primary efficacy outcome) occurred in 261 patients (2.4%) (Online Figure 1A). Patients treated with durable polymer PtCr-EES had a risk of TLR comparable to that observed with other DES (2.2% versus 2.6%; OR 0.98, 95% CI: 0.75-1.29, p=0.90; I2=9%, p for heterogeneity - phet=0.36). Of interest, in those trials with protocol-mandated control angiography9,11, the degree of in-stent late lumen loss was comparable between durable polymer PtCr-EES and other DES (weighted mean difference 0.02, 95% CI: -0.05, 0.09, p=0.52; I2=0%, phet=0.50; data available for 475 patients).
Definite/probable ST (primary safety outcome) occurred in 71 patients (0.6%) (Online Figure 1B). Patients treated with durable polymer PtCr-EES had a risk of definite/probable ST comparable to that observed with other DES (0.6% versus 0.7%; 0.89 [0.55-1.45], p=0.63; I2=0%, phet=0.56). The risk of early (0.2% versus 0.5%; 0.46 [0.19-1.11], p=0.08; I2=0%, phet=0.62) and late (0.1% versus 0.1%; 1.66 [0.23-12.05], p=0.61; I2=23%, phet=0.26) definite/probable ST was comparable between durable polymer PtCr-EES and other DES (data available for 5,851 participants).
Definite ST occurred in 44 patients (0.4%). Patients treated with durable polymer PtCr-EES had a risk of definite ST comparable to that observed with other DES (0.4% versus 0.5%; 0.96 [0.53-1.76], p=0.90; I2=0%, phet=0.88). The risk of early (0.2% versus 0.3%; 0.93 [0.34-2.49], p=0.88; I2=0%, phet=0.42) and late (0.2% versus 0.2%; 0.98 [0.43-34.55], p=0.98; I2=25%, phet=0.28) definite ST was comparable between durable polymer PtCr-EES and other DES (data available for 7,801 participants).
MI occurred in 244 patients (2.3%) (Online Figure 2A). Patients treated with durable polymer PtCr-EES displayed a risk of MI comparable to that observed with other DES (2.2% versus 2.4%; 1.05 [0.81-1.38], p=0.70; I2=0%, phet=0.43). Of interest, MI related to the target vessel occurred in 130 patients (1.3%, data available for 10,010 participants). Patients treated with durable polymer PtCr-EES had a risk of MI related to the target vessel comparable to that observed with other DES (1.2% versus 1.7%; 0.97 [0.59-1.61], p=0.91; I2=44%, phet=0.15).
TVR occurred in 368 patients (3.5%, data available for 10,510 participants) (Online Figure 2B). Patients treated with durable polymer PtCr-EES displayed a risk of TVR comparable to that observed with other DES (3.3% versus 3.8%; 1.04 [0.79-1.38], p=0.78; I2=33%, phet=0.19).
Death occurred in 259 patients (2.4%) (Online Figure 3A). Patients treated with durable polymer PtCr-EES displayed a risk of death comparable to that observed with other DES (2.3% versus 2.5%; 1.00 [0.77-1.30], p=0.99; I2=0%, phet=0.44). Of interest, cardiac death occurred in 152 patients (1.4%) (Online Figure 3B). Patients treated with durable polymer PtCr-EES displayed a risk of cardiac death comparable to that observed with other DES (1.3% versus 1.6%; 0.86 [0.61-1.19], p=0.36; I2=0%, phet=0.88).
LSD occurred in 16 patients (0.2%, data available for 7,372 participants) (Online Figure 3C). Durable polymer PtCr-EES were associated with a higher risk of LSD as compared to other DES (0.4% versus 0%; 12.05 [1.60-90.71], p=0.02; I2=0%, phet=0.65). However, the higher risk of LSD with durable polymer PtCr-EES did not result in a higher risk of TLR (p for interaction - pint=0.41) or definite/probable ST (pint=0.72) as compared to other DES. A detailed description of clinical, angiographic and procedural features as well as of subsequent clinical outcomes of patients with LSD has been provided in Online Table 4.
SMALL STUDY EFFECTS, INFLUENCE AND SENSITIVITY ANALYSES
Funnel plot distribution of primary efficacy and safety outcomes was derived from the standard error of the natural logarithm OR plotted against the OR of TLR and definite/probable ST, respectively (Online Figure 4A, Online Figure 4B). Of note, the absence of bias due to small study effects was confirmed both visually and mathematically. Additionally, influence analysis demonstrated that no single study significantly altered the summary OR for primary outcomes (Online Table 5). The trial sequential analysis revealed that the accumulated sample size provided robust evidence for the primary efficacy outcome, but was still inadequate for the primary safety outcome (Online Figure 5A, Online Figure 5B). Risk estimates for both TLR and definite/probable ST were independent from the comparator DES (pint≥0.29), the polymer releasing the antirestenotic drug (pint≥0.21) or the strut thickness (pint≥0.27) in the control DES group, the all-comers design of the trials (pint≥0.08) or the protocol-mandated control angiography (pint≥0.21) (Online Figure 6A, Online Figure 6B).
Discussion
We undertook this meta-analysis to investigate the outcomes of PCI patients treated with either durable polymer PtCr-EES or other new-generation DES. At one-year follow-up, the durable polymer PtCr-EES showed: (i) similar efficacy and safety though (ii) a higher susceptibility to longitudinal deformation as compared to other DES.
The use of platinum as an alloy compound was aimed at improving the mechanical properties of metallic stent backbones5. Although preclinical studies of PtCr-based platforms have demonstrated less thrombogenicity and favourable endothelialisation patterns5,6, these qualities might be difficult to measure in patients, because differences between devices that are detectable at bench level might have no clinical counterpart in vivo27.
The durable polymer PtCr-EES investigated in the present meta-analysis consists of a PtCr metallic scaffold (81 µm strut thickness) coated with a poly n-butyl methacrylate primer layer and a durable drug matrix layer composed of a copolymer of polyvinylidene fluoride and hexafluoropropylene blended with everolimus (100 μg/cm2 stent surface). Apart from the metallic scaffold, the drug/polymer combination of this device has the same biological behaviour as the CoCr-EES platform, which represents a benchmark new-generation DES28.
Randomised clinical trials8-14 investigating the comparative efficacy of durable polymer PtCr-EES against other new-generation DES platforms lacked the statistical power to draw firm conclusions regarding clinical outcomes. With this in mind, and considering the number of ongoing, large-scale randomised trials aiming to address this issue (NCT02193971, NCT01979744, NCT01740479, NCT01347554), we conducted a meta-analysis to investigate the performance of durable polymer PtCr-EES in PCI patients. By aggregating the data from seven randomised trials with ≈11,000 PCI patients allocated to durable polymer PtCr-EES versus other DES, this is the most comprehensive meta-analysis dealing with this topic. Previous meta-analyses did not include all randomised trials comparing the durable polymer PtCr-EES versus new-generation DES28, evaluated mixed PtCr-EES platforms with different polymer coatings29, and based their conclusions predominantly on indirect comparisons of PtCr-EES with early- and new-generation DES platforms30.
First, in this report patients treated with the durable polymer PtCr-EES showed a risk of target lesion and target vessel revascularisation comparable to that of patients treated with other DES at one-year follow-up. Neither stent- nor trial-related features influenced the risk estimation of repeat revascularisation. On the one hand, this result confirms the favourable antirestenotic efficacy of durable polymer PtCr-EES observed in large-scale registries of unselected patients31,32, and corroborates the angiographic findings of those trials9,11 with available invasive surveillance. On the other hand, we showed for the first time that the data accumulated in >10,000 PCI patients provides robust evidence to discard a clinically relevant benefit in terms of repeat revascularisation associated with durable polymer PtCr-EES.
Second, this meta-analysis found a comparable risk of ST for durable polymer PtCr-EES and other DES. The risk estimation for ST was independent from stent- and trial-related features. The present analysis confirms once more the excellent safety profile of current high-performance DES, with an overall rate of ST after 12 months of 0.6% among >10,000 patients. The overall risk of ST appeared low and comparable among DES platforms studied, even though a large proportion of participants were enrolled in trials with an “all-comers” design8,9,12. Notwithstanding this, the impact of durable polymer PtCr-EES on ST needs to be studied further, since the available sample size accounts for <20% of that required to address a measurable effect of this device with respect to this endpoint. Ongoing large-scale randomised trials remain instrumental to disclose whether the mechanical and biological properties of PtCr-based stent platforms may lead to a lower thrombotic risk in humans.
Finally, the durable polymer PtCr-EES studied in this report carried a 12-fold increase in the risk of longitudinal deformation as compared with other DES. The occurrence of LSD remained negligible in absolute terms and was not associated with a worse clinical prognosis. Although instances of distortion or shortening of a stent along the longitudinal axis following its successful deployment are described with all DES platforms33, in recent years the majority of cases of LSD have involved the durable polymer PtCr-EES studied in this report. If unrecognised or left untreated, LSD has occasionally been associated with acute MI, ST and worse prognosis33. The incidence of LSD observed in the current analysis is somewhat lower than that reported in other series34,35. The lack of systematic use of intracoronary imaging, the different definitions of LSD applied in the original trials (ranging from changes in radiopacity to longitudinal shortening), and the relatively favourable lesions treated, may possibly have led to underreporting. Notably, the durable polymer PtCr-EES investigated in this study has been subject to iterations in the frame design (more connectors between the proximal hoops), which reduce the risk of LSD36. In this regard, our findings should be restricted to PCI patients treated with the original durable polymer PtCr-EES configuration, and with clinical and angiographic features similar to those reported in the present analysis.
Study limitations
A number of limitations inherent to the present study should be discussed. First, this meta-analysis was based on aggregate data and shared the flaws of the original trials. Second, the median follow-up was limited to one year and a longer follow-up is certainly desirable in comparative studies, especially to disclose fully significant differences in terms of safety outcomes. Third, different stent platforms with various combinations of drugs, polymers and backbones were represented in the control group, a fact that weakens the weight of the final results. Despite this issue having been addressed by undertaking additional analyses based on comparison against each of the individual control stents, the complex interplay between main DES components and clinical outcomes cannot be fully disclosed in the context of this study.
Fourth, this report cannot exclude that the outcomes associated with durable polymer PtCr-EES versus other new-generation DES may differ in specific subgroups of lesions and patients. Fifth, the type and the length of dual antiplatelet therapy differed among the trials included, and the actual compliance to prescribed antithrombotic therapy was not routinely reported within the original trials. Finally, the protocol-mandated surveillance angiography in two trials9,11 may have magnified differences in the absolute proportion of revascularisations across groups. However, relative differences are unlikely to have been affected.
Conclusions
At one-year follow-up, clinical outcomes of PCI patients treated with durable polymer PtCr-EES are comparable to those of patients treated with other new-generation DES. The risk of stent thrombosis with durable polymer PtCr-EES as compared to other new-generation DES requires further investigation. The higher susceptibility to longitudinal deformation associated with durable polymer PtCr-EES remains rare in absolute terms and does not lead to clinical sequelae.
| Impact on daily practice Innovative components and thinner strut designs are the mainstay of contemporary drug-eluting stent technology. Stents eluting everolimus from a thin platinum-chromium metallic platform are used widely in daily practice. This analysis found that durable polymer platinum-chromium everolimus-eluting stents are associated with similar clinical outcomes but inferior longitudinal stability as compared to other modern drug-eluting stents. Despite the fact that drug-eluting stents with thinner-strut design represent an attractive alternative for challenging coronary anatomies, the trade-off in terms of longitudinal stability associated with these devices should be considered when selecting the best platform to be implanted. |
Conflict of interest statement
A. Kastrati reports patent applications related to drug-eluting stent technologies. R. Byrne reports receiving lecture fees from B. Braun Melsungen AG, Biotronik and Boston Scientific and scientific support from Boston Scientific and HeartFlow. R. Colleran reports support from the Irish Board for Training in Cardiovascular Medicine sponsored by MSD. D. Giacoppo is the recipient of a research fellowship grant funded by the European Association of Percutaneous Cardiovascular Interventions (EAPCI). The other authors have no conflicts of interest to declare.
Supplementary data
Online Appendix. Search strategy and selection criteria
Online Table 1. Main characteristics of trials included in the study.
Online Table 2. Definitions of clinical outcomes according to protocols within trials included in the study.
Online Table 3. Assessment of risk of bias.
Online Table 4. Main characteristics and clinical outcomes of patients with LSD as reported in the original trials.
Online Table 5. Influence analysis of primary outcomes.
Online Figure 1. Risk estimates of primary outcomes.
Online Figure 2. Risk estimates of secondary outcomes (MI, TVR).
Online Figure 3. Risk estimates of secondary outcomes (death, cardiac death, LSD).
Online Figure 4. Funnel plot distribution of trials according to primary outcomes.
Online Figure 5. Trial sequential analysis for primary outcomes.
Online Figure 6. Sensitivity analysis for primary outcomes.
Supplementary data
To read the full content of this article, please download the PDF.

