Abstract
Aims: Because of the reduction in the rate events related with in-stent restenosis, most events after drug-eluting stent implantation occur shortly after coronary stenting. Cobalt-chromium alloys allow to reduce strut thickness and improve flexibility and deliverability of coronary stent platforms, and thus could be associated with lower short-term events after stenting. The aim of this study was to test the hypothesis that drug-eluting coronary stents with a cobalt-chromium platform reduce the incidence of periprocedural (30-day) myocardial infarction in comparison with stainless steel drug-eluting coronary stents.
Methods and results: A meta-analysis from nine randomised trials comparing cobalt-chromium and stainless steel drug-eluting coronary stents that overall included 11,313 patients was performed. The incidence of myocardial infarction, stent thrombosis, and cardiac death at 30 days was compared between both types of stents. At 30 days, the incidence of acute myocardial infarction was significantly lower in patients allocated to cobalt-chromium drug-eluting stents (2.3% vs. 3.9%, respectively; p=0.006; odds ratio 0.72, 95% confidence interval 0.58–0.91), due to a significant reduction in the rate of non-Q-wave myocardial infarction (odds ratio 0.67, 95% confidence interval 0.51–0.88). The incidence of stent thrombosis was similar between both groups of patients, (0.5% vs. 0.5%, p=0.76; odds ratio 1.09, 95% confidence interval 0.63–1.89).
Conclusions: Drug-eluting coronary stents that use cobalt-chromium stent platforms have a better safety profile at 30 days in comparison with stainless steel drug-eluting stents, due to a significant reduction in the rate of myocardial infarction.
Introduction
Sirolimus-eluting Cypher stent and paclitaxel-eluting Taxus stent have been extensively used worldwide because of their widely proven clinical benefit in comparison with bare-metal stents in very different clinical and angiographic scenario1,2. These drug-eluting stents (DES) have a polymer release of antiproliferative drugs from a stainless steel stent platform.
More recently, second generation DES have been developed, mainly to improve long-term DES safety but also to facilitate the procedure by using more flexible and deliverable stents. In some cases, second generation DES use cobalt-chromium alloy stent platforms. Cobalt-chromium (CC) is stronger and more radio-opaque than stainless steel (SS), and thus allows to reduce strut thickness and total stent volume while maintaining radial strength leading to more flexible stent platforms3. Because of that, cobalt chromium stents have an improved deliverability and reduce damage to the vessel wall in comparison with stainless steel coronary stents.
Although infrequent, late and mainly very late, stent thrombosis have received special attention when evaluating safety of DES4. However, due to the dramatic reduction in the need for new revascularisation procedures secondary to restenosis, most major cardiac events after DES implantation occur during in the periprocedural period (first month after stent implantation)5,6. Because of that, we considered of great interest to focus on early safety of different types of DES accordingly to the stent platform used, specifically on the potential clinical benefits of using a cobalt-chromium stent platform.
The hypothesis of the study was that second-generation DES that use cobalt-chromium stent platforms are safer at short-term follow-up than stainless steel DES, by reducing periprocedural myocardial infarctions. To test this hypothesis, we performed a meta-analysis from nine randomised clinical trials that compared stainless steel DES with cobalt-chromium DES. Clinical events at 30 days were compared between both types of DES5-15.
Methods
Trials included in the meta-analysis
A computer-based search was done to identify randomised trials that compared cobalt-chromium DES with stainless steel DES. MEDLINE (PUBMED), as well as the website of major scientific meetings was searched (Paris Coronary Revascularisation course, Transcatheter and Cardiovascular Interventions, Congress of the European Society of Cardiology, and Scientific Sessions of the American Heart Association, and American College of Cardiology). Trials that were performed in the setting of primary percutaneous coronary intervention in the setting of ST-segment elevation acute myocardial infarction were excluded. Finally, 11 trials were identified5-15. At the time of this manuscript was written, no data on 30-day incidence of myocardial infarction was available in the SPIRIT-IV and ISAR-TEST-2, and thus these two trials were not included in this meta-analysis. The remaining nine trials were included in this work. Overall, 11,313 patients were included in these trials. Table 1 shows the trials included in the meta-analysis.
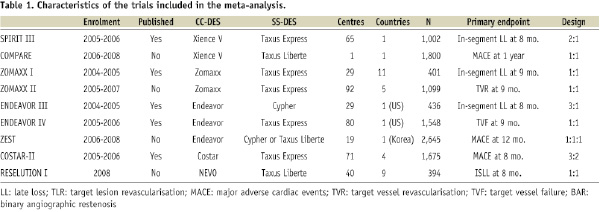
All the trials included were single-blind. The ZOMAXX-trial was early interrupted by the sponsors. The COMPARE trial was a single-centre trial. The rest of the trials were multicentre. ZOMAXX-I, ZOMAXX-II, COSTAR-II, and RES-ELUTION trial were international studies, whereas SPIRIT-III, ENDEAVOR-III, ENDEAVOR-IV (US), COMPARE (The Netherlands), and ZEST (Korea) studies were done in a single country.
Devices evaluated in the trials
Table 2 shows the characteristics (e.g., stent platform, strut thickness) of the different DES used in the trials: Cypher (Cordis Corp., Miami Lakes, FL, USA), Taxus express and Taxus Liberte (Boston Scientific Corp., Natick, MA, USA), Endeavor (Medtronic Vascular, Inc., Santa Rosa, CA, USA), Xience V (Abbott Vascular, Santa Clara, CA, USA), Costar (Conor Medisystems, Menlo Park, CA, USA), NEVO (Cordis Corp., Miami Lakes, FL, USA), and Zomaxx (Abbott Vascular, Santa Clara, CA, USA).
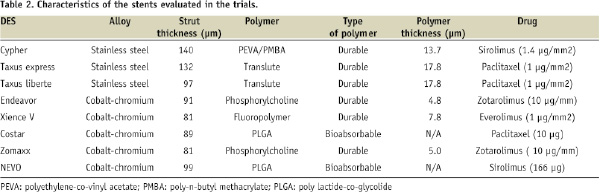
The CC-DES was the everolimus-eluting XienceV stent in the SPIRIT-III and COMPARE trials. The following trials evaluated zotarolimus-eluting stents: ZOMAXX-I, ZOMAXX-II, ENDEAVOR-III, ENDEAVOR-IV, and ZEST. The CC-DES was the NEVO coronary stent in the RES-ELUTION-I trial, and the Costar stent in the COSTAR-II study. Stainless steel DES was the Taxus coronary stent and the Cypher coronary stent in most of the trials. The ZEST study compared one cobalt-chromium stent with two different types of stainless steel DES.
Outcomes and definitions
The following outcomes were studied at 30 days after procedure: cardiac death, acute myocardial infarction (AMI), and stent thrombosis. Myocardial infarction was defined in most trials as the development of new pathological Q-waves 0.4 seconds or longer in duration in two or more continuous leads, or CPK elevation of at least 2-fold the upper normal limit with positive levels of CPK-MB (SPIRIT –III, ZOMAXX-I, ENDEAVOR-III). In the ZEST trial, however, a CPK elevation of ≥3 times the upper normal limit was required.
When provided (the majority of the trials), the rate of stent thrombosis accordingly to the ARC criteria, was used16. Some trials (COSTAR-II, ZOMAXX-I) provided only the rate of stent thrombosis following a per-protocol definition (“abrupt vessel closure resulting in clinical manifestations of ischaemia and angiographic evidence of occlusion or flow-limiting thrombosis in a treated vessel, in which the investigational device was successfully implanted”; or “acute coronary syndrome with angiographic evidence of thrombus within or adjacent to a previously treated lesion or, in the absence of angiography, any unexplained death or AMI with ST segment elevation or new Q-waves in the distribution of the target lesion occurring within 30 days”).
In most trials, device success was defined as a result with <50% (ENDEAVOR-III, RES-ELUTION-I) or <30% residual stenosis was obtained after stent implantation. Procedural success was defined as a device success without in-hospital major adverse cardiac events.
Procedural details
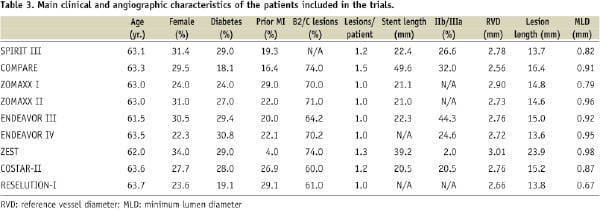
In many trials, very complex lesions (ostial lesions, left main lesions, bifurcations involving a ≥2 mm side branch, saphenous vein grafts, in-stent restenosis, severely calcified lesions, or vessels with sever tortuosity, total occlusion, or thrombus-containing lesions) were excluded (SPIRIT-III, ZOMAXX-I, ZOMAXX-II, ENDEAVOR-III, RES-ELUTION-I, COSTAR-II), but some studies included ostial lesions, restenosis, severely calcified lesions, bifurcations, and lesions located at left main or at a coronary bypass (ZEST,COMPARE). In fact, the proportion of patients with type B2/C lesions was ~ 2/3 in most of the studies (Table 3).
Many of the trials included required predilatation (SPIRIT-III, ZOMAXX-I, ENDEAVOR-III, COSTAR-II), but other allowed direct coronary stenting. For example, direct stenting was performed in 8% of patients included in the ZEST study, and in 34% of the COMPARE trial patients.
Anticoagulation and antiplatelet therapy
In the majority of the trials, un-fractionated heparin was used during procedure at a dose of 100 IU/Kg to maintain an activating clotting time (ACT) ≥250 seconds (200-250 seconds when IIb/IIIa inhibitors were used). In the SPIRIT III study the use of bivalirudin instead of unfractionated heparin was allowed. The use of IIb/IIIa inhibitors ranged from 2% to 44%.
Double therapy with aspirin plus clopidogrel was the default antiplatelet regimen in all the trials. The initial dose of aspirin before the procedure varied among the trials (e.g., ≥100 mg in ZEST, ≥300 mg in SPIRIT-III, 325 mg in ENDEAVOR studies, and ≥325 mg in COSTAR-II). The loading dose of clopidogrel was more uniform among the studies (300 or ≥300 mg in most of the studies), that was recommended before the procedure in most of them. The daily dose of aspirin after stenting also varied among the studies (e.g., ≥80 mg in SPIRIT-III, 100-325 mg in ZEST, 325 mg in COSTAR-II). The dose of clopidogrel after stenting, however, was 75 mg/d in all the trials. The duration of treatment with clopidogrel was three months (ENDEAVOR-III), ≥6 months (SPIRIT-III, ENDEAVOR-IV, COSTAR-II, RES-ELUTION-I), 12 months (COMPARE), or ≥12 months (ZEST).
Statistical analysis
The review was conducted according to the Quality of Reports of Meta-Analyses of Randomized Clinical Trials (QUOROM) recommendations17. The Reviewer Manager 5.1 (2000 Cochrane Collaboration) and the SPSS 10.0 (SPSS Inc., Chicago, IL, USA) statistical packages were used.
Quantitative variables are expressed as mean values, and discrete variables as percentages. Funnel plots were also constructed to rule out a potential bias in the selection of the trials. The odds ratio (OR) for acute myocardial infarction, cardiac death, and stent thrombosis at 30 days, and their 95% CI were calculated comparing CC-DES with SS-DES rates using raw data for each study and for the pooled population (intention-to-treat basis). The fixed-effect model or the Der Simonian and Laird random-effect model (when p<0.05 for Q-test for heterogeneity) were used. The combined effect for the heterogeneity was calculated by taking the inverse variance estimated.
Results
Characteristics of the trials included
Table 1 shows the characteristics of the trials, such as the number of patients included, the number of participating centres and countries, and the stents that were evaluated in each of the studies. Six of the trials had a clinical primary endpoint (mainly major adverse cardiac events, but also target vessel failure, target vessel revascularisation, and target lesion revascularisation at follow-up), and five had an angiographic primary endpoint (mainly in-segment late loss, but also in-stent late loss, and the rate of binary angiographic restenosis).
Table 3 shows the main clinical and angiographic characteristics of the study population of each trial. The percentage of patients with diabetes ranged between 18% and 29%, and the proportion of female patients ranged from 22% to 31%. The percentage of patients with lesions type B2 or C was ~ 2/3.
Rate of myocardial infarction at 30 days
Figure 1 shows the funnel plot for the rate of 30-day myocardial infarction, showing absence of asymmetry and thus absence of evidence of selection bias.
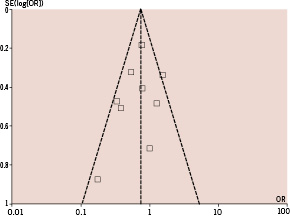
Figure 1. Funnel plot of the trials comparing the rate of myocardial infarction at 30 days between patients allocated to CC-DES, and SS-DES.
Figure 2 shows the forest plot including the OR as well as the 95% CI for the rate of myocardial infarction at 30 days in each of the studies as well as in all the trials.
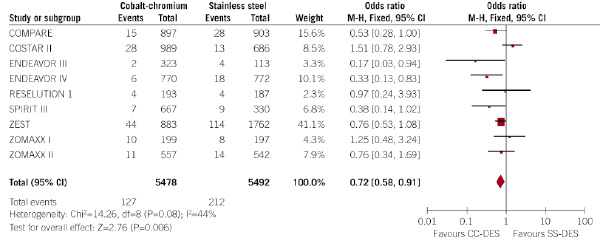
Figure 2. Evaluation of the influence of the type of DES (CC-DES or SS-DES) on the incidence of myocardial infarction at 30 days (forest plot).
Patients allocated to CC-DES had a significantly lower rate of myocardial infarction at 30 days (2.3% vs. 3.9%, respectively; p=0.006; odds ratio 0.72, 95% confidence interval 0.58 – 0.91). That means a relative risk reduction of 41%, and an absolute risk reduction of 1.6%, in the rate of myocardial infarction at 30 days.
Q-test for heterogeneity showed no significant heterogeneity among the trials. However, as Q-test for heterogeneity was 0.08, a random-effect model was also performed, and results did not differ (odds ratio 0.69, 95% confidence interval 0.48 – 0.99; p=0.04), thus confirming the lower incidence of myocardial infarction at 30 days in patients allocated to CC-DES.
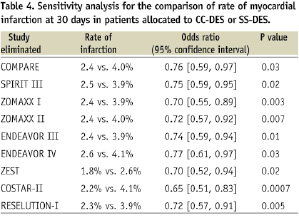
Table 4 shows the sensitivity analysis for the influence of the type of DES on the rate of myocardial infarction at 30 days.
Six trials provided the rate of Q-wave and non-Q-wave myocardial infarction at 30 days (Figures 3 and 4).
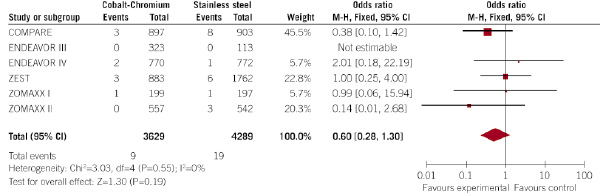
Figure 3. Evaluation of the influence of the type of DES (CC-DES or SS-DES) on the incidence of Q-wave myocardial infarction at 30 days (forest plot).
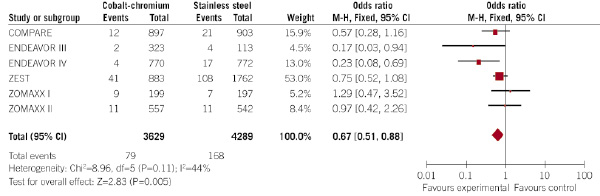
Figure 4. Evaluation of the influence of the type of DES (CC-DES or SS-DES) on the incidence of non-Q-wave myocardial infarction at 30 days (forest plot).
Interestingly, the rate of Q-wave infarction was not statistically different between CC-DES and SS-DES (0.2% vs. 0.4%, respectively; p=0.19; OR 0.60, 95% CI 0.2 –1.30), but the rate of non-Q-wave myocardial infarction was significantly lower in patients allocated to CC-DES (2.2% vs. 3.9% in patients allocated to SS-DES; p=0.005; OR 0.67, 95% CI 0.51 – 0.88). No significant heterogeneity among the trials was observed.
Rate of death and stent thrombosis at 30 days
All the trials provided the rate of stent thrombosis at 30 days. Figure 5 shows the forest plot for the effect of the type of DES over the incidence of stent thrombosis at 30 days.
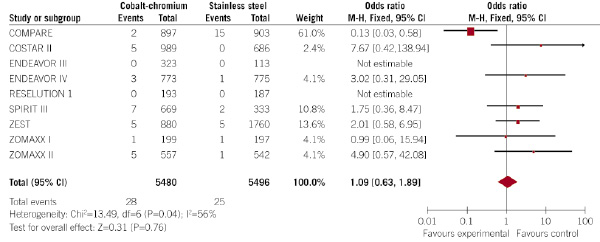
Figure 5. Evaluation of the influence of the type of DES (CC-DES or SS-DES) on the incidence of stent thrombosis at 30 days (forest plot).
Stent thrombosis occurred in a similar rate in patients allocated to CC-DES and SS-DES (0.5% vs. 0.5%, respectively; p=0.76; OR 1.09, 95% CI 0.63 – 1.89).
Similar findings occurred with the rate of cardiac death, that occurred in a similar proportion of patients allocated to CC-DES and SS-DES (0.2% vs. 0.2%, respectively; p=0.33; OR 1.52, 95% CI 0.66 – 3.55) (Figure 6).
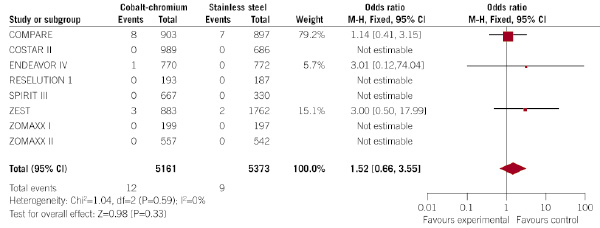
Figure 6. Evaluation of the influence of the type of DES (CC-DES or SS-DES) on the incidence of cardiac death at 30 days (forest plot).
Discussion
With the generalisation of DES, long-term safety of these devices (mainly late stent thrombosis) has received great attention18. However, due to the dramatic reduction in the rate of restenosis and need for new revascularisation procedures, most events after DES implantation occur within the first month after stent implantation4,5. Even, some data have shown that DES may reduce the incidence of non-Q-wave acute myocardial infarction at one year in comparison with bare-metal stents19. Because of that, when comparing different types of DES, short-term safety is becoming crucial. For example, in the recently presented SPIRIT-III, and COMPARE randomised trials, >2/3 of cardiac death, myocardial infarction, and stent thrombosis occurring during the first year after stenting took place within the first month4,5.
In our meta-analysis, all patients were treated with DES, but some were allocated to CC-DES and other to SS-DES. The most important finding of our study was that patients allocated to CC-DES had a better safety profile at 30 days, showing a significantly lower incidence of myocardial infarction at 30 days, from 3.9% to 2.3%. That means a relative risk reduction of ~40%, and an absolute risk reduction of 1.6%. Thus, the number of patients needed to be treated with a CC-DES instead of a SS-DES was 63. The incidence of stent thrombosis and cardiac death, however, was similar between both groups of patients.
The reduction in the rate of myocardial infarction at 30 days was due to a significant reduction in the rate of non-Q-wave myocardial infarction. In the trials that provided the rate of Q-wave and non-Q-wave myocardial infarction separately, the incidence of Q-wave myocardial infarction was not different with both types of DES, but non-Q-wave myocardial infarction occurred significantly less frequently in patients allocated to CC-DES (2.2% vs. 3.9%). This occurred in spite the fact that in some trials (e.g., SPIRIT-III, ZOMAXX-II) patients allocated to CC-DES received more stents and more stent length in comparison with SS-DES. Also of interest, the proportion of patients with type B2 or C lesions was similar in patients allocated to CC-DES and SS-DES in most trials, except in the ENDEAVOR-III, that found more type B2/C lesions in patients allocated to CC-DES.
Periprocedural myocardial infarction after coronary stenting may be secondary to distal embolisation of microscopic particles that may even be not apparent at angiography. Other potential explanations for myocardial infarction shortly after stenting are abrupt closure (even transient during the procedure), side branch compromise, tissue prolapse, coronary spasm, flow-limitating coronary dissection, non-reflow phenomenon, and early stent thrombosis21,22. Clinical predictors for myocardial infarction after percutaneous coronary interventions are multivessel disease, lesion length, complex lesions, presence of thrombus, lesions located at saphenous vein grafts, acute coronary syndromes, and use of IIb/IIIa inhibitors21,22. Periprocedural non-Q-wave myocardial infarction after percutaneous coronary interventions is of clinical importance, because it is associated with long-term clinical outcome23,24.
Cobalt-chromium alloys, due to its mechanical characteristics, allow to reduce strut thickness and improve stent designs maintaining radial strength and radio-opacity in comparison with traditional SS stent platforms. Because of that, CC stent platforms have thinner struts and improved flexibility, deliverability and vessel conformity, and thus may produce less vessel injury during the procedure and therefore they could reduce the rate of distal embolisation and edge-dissections25-27. In a study with optical coherence tomography, CC-DES were associated with better stent performance to the vessel wall28. Other potential explanation for the reduction in the rate of periprocedural infarction with the use of CC-DES is a higher rate of immediate device success. For example, in the ENDEAVOR-III trial, the rate of immediate device success was significantly higher with CC-DES as compared to SS-DES (99% vs. 95%, p=0.02). Additionally, less frequent side branch compromise, and better stent performance when overlapping stents, could have contributed to the lower rate of myocardial infarction in CC-DES patients.
Study limitations
This study has some limitations. First, as with other meta-analyses, inclusion criteria may be different among studies. Second, the potential role of various polymers and drugs used in different DES in the rate of periprocedural infarction has not been evaluated in this study. Third, the exact mechanism of myocardial infarctions was not provided in each study. Four, in most of the trials, complex lesions such as bifurcations were excluded. This may have underestimated the beneficial effect of CC-DES in the rate of periprocedural complications in the real world. Finally, antithrombotic treatments may have played a role in the rate of periprocedural myocardial infarction. However, although some treatments differed among the trials, in all the trials included, patients allocated to CC-DES and SS-DES received a similar pharmacologic treatment.
Conclusions
In this meta-analysis comparing CC-DES and SS-DES, CC-DES were associated with a significantly lower incidence of myocardial infarction at 30 days. This was due to a significant reduction in the rate of non-Q-wave myocardial infarctions in this time period, and although several potential explanations may be involved, a less vessel damage due to the thinner struts and better stent platforms when using this alloy is probably the main reason for this clinical benefit. These findings are crucial in the study design, and interpretation of upcoming head-to-head trials comparing different types of DES.

