Abstract
Aims: To establish whether technological improvements in drug-eluting stent (DES) technology introduced in second-generation (G2) DES have contributed to improving patient-focused outcomes.
Methods and results: We performed a systematic review of randomised clinical trials (RCT) comparing first-generation (G1) and G2 DES with a >9-month clinical follow-up. The primary endpoint for efficacy was ischaemia-driven target lesion revascularisation (ID-TLR); safety endpoints were all-cause death, myocardial infarction (MI) and stent thrombosis (ST). Sixteen RCTs involving 25,427 patients met eligibility criteria (17 comparisons). In these trials, paclitaxel (PES) and sirolimus (SES) were compared with everolimus (EES), zotarolimus (ZES) or biolimus A9 (BES) DES. G2 varied in metal alloy, strut thickness and type of drug-eluting matrix. Overall, G2 DES were associated with a 26% relative risk reduction (RRR) of MI (relative risk [RR]=0.74, 95% CI: 0.61-0.90, p=0.003) and ST (RR=0.70, 95% CI: 0.55-0.89, p=0.004), while no significant benefit was observed for ID-TLR and death. Use of 2G DES was associated with a significant reduction in the risk of ID-TLR (RR=0.66, 95% CI: 0.51-0.85, p=0.002), MI (RR=0.60, 95% CI: 0.49-0.72, p<0.001) and ST (RR=0.41, 95% CI: 0.26-0.65, p=0.001) when compared with PES. Strut thickness ≤91 µm in G2 DES was associated with a significantly lower risk of MI (RR=0.54, 95% CI: 0.51-0.86, p=0.002).
Conclusions: The introduction of thinner stent struts and other technological improvements made in G2 DES technology have translated into better patient outcomes. Overall, the net benefit of G2 DES over G1 DES is expressed in terms of ID-TLR and ST risk reduction but it could be masked by heterogeneities in the use of G1 comparators and the use of non-inferiority study designs in RCTs.
Abbreviations
BES: biolimus-eluting stent
BMS: bare metal stent
CAD: coronary artery disease
DES: drug-eluting stent
EES: everolimus-eluting stent
G1 DES: first-generation drug-eluting stent
G2 DES: second-generation drug-eluting stent
ID-TLR: ischaemia-driven target lesion revascularisation
MI: myocardial infarction
NNT: number needed to treat
PES: paclitaxel-eluting stent
RCT: randomised clinical trial
RR: relative risk
RRR: relative risk reduction
SES: sirolimus-eluting stent
ST: stent thrombosis
ZES: zotarolimus-eluting stent
Introduction
Drug-eluting stents (DES) have contributed to the widespread use of percutaneous coronary revascularisation, following the demonstration that they significantly reduce restenosis rates1. Most of the available information on DES efficacy and safety stems from randomised clinical trials using the sirolimus-eluting stent CYPHER® (Cordis, Johnson & Johnson, Warren, NJ, USA) and the paclitaxel-eluting stent TAXUS™ Express2™ (Boston Scientific, Natick, MA, USA)2. The enthusiasm generated by these first-generation (G1) DES has been tempered by growing evidence revealing that DES would not abolish restenosis, but rather would reduce its occurrence1. Furthermore, the demonstration of a higher prevalence of late and very late stent thrombosis in DES, compared with BMS, became a new matter of concern for interventionalists3. With the aim of overcoming these problems, newer DES designs introduced changes both in the structural characteristics of the stent platform and in the antiproliferative cover (eluting polymer and drug). Since second-generation (G2) DES have been tested individually against a pre-existing G1 DES in non-inferiority trials for their approval by regulatory agencies, the overall clinical benefit of G2 DES has not been explored.
With this aim, we performed a systematic review and meta-analysis of the available clinical trials comparing G1 and G2 DES, paying attention to the clinical benefit and to the effect of individual modifications made in DES technology.
Methods
The study was performed in compliance with the Cochrane Handbook for Systematic Reviews of Interventions and the recommendations set forth by the Quality of Reporting of Meta-Analysis (QUORUM)4,5. First-generation DES were the sirolimus-eluting stent CYPHER® and the paclitaxel-eluting stent TAXUS™. Versions of the CYPHER (CYPHER® and CYPHER SELECT®) and TAXUS (TAXUS™ Express2™ and TAXUS® Liberté®) were included as G1 DES on the grounds that no significant changes in alloy, strut thickness, eluting polymer or drug were introduced. Second-generation DES were defined as those introduced later with modifications in antiproliferative drug, eluting polymer or stent platform.
SEARCH STRATEGY
An electronic search for trials comparing G1 and G2 DES was performed by two independent investigators in TripDatabase, PubMed, Cochrane Central Register of Controlled Trials, abstract databases from relevant scientific congresses (American College of Cardiology, American Heart Association, European Society of Cardiology, Transcatheter Cardiovascular Therapeutics and EuroPCR) and internet-based sources of information on cardiology (cardiosource.com, escardio.org, tctmd.com, the heart.org and ctronline.com). The search period was January 2003 to September 2011. Appropriate medical subjects heading (MeSH) terms and free text words including “stent”, “drug-eluting stent”, “sirolimus-eluting stent”, “paclitaxel-eluting stent”, “everolimus-eluting stent”, “biolimus-eluting stent”, “zotarolimus-eluting stent” , “coronary artery disease”, and “randomised clinical trial” were used. In addition, relevant reviews and earlier meta-analyses were also reviewed for potential studies. Principal investigators of trials were contacted whenever required to provide missing data from presentations as Late Breaking Trials in the above conferences and still in press. No language restrictions were enforced.
CRITERIA FOR STUDY SELECTION
Studies were included if they: 1) compared G1 and G2 DES using a randomised design and used ischaemia-driven target lesion revascularisation as efficacy endpoint; and 2) reported on outcomes of interest during a follow-up period ≥9 months. Randomised comparisons between G1 versus G2 DES exclusively addressing subgroups were excluded.
Inclusion or exclusion of studies was performed hierarchically, based on the title of the report first, followed by the abstract and then by the full text. Consulting a third investigator solved disagreement on study selection.
OUTCOME AND CLINICAL DEFINITIONS
The primary efficacy endpoint of interest was ischaemia-driven target lesion revascularisation (referred to hereafter as ID-TLR), and defined as any revascularisation procedure (percutaneous or surgical) involving the target lesion owing to significant luminal renarrowing in the presence of symptoms or objective signs of ischaemia. The endpoints of all death and myocardial infarction were defined according to definitions proposed by Cutlip et al for coronary stent trials6. Stent thrombosis was adjudicated according to the criteria for definite or probable stent thrombosis of the Academic Research Consortium6. Possible STs were not considered.
DATA ABSTRACTION
Two independent investigators using pre-specified standardised forms independently extracted data. Data extracted included: trial name, publication year, sample size and characteristics, study design, stent type characteristics, type of intervention (number of patients and lesions allocated to G1 or G2 DES), length of follow-up, presence of mandated angiographic follow-up, minimal length of dual antiplatelet therapy, outcome measures and primary endpoints. In case of studies reporting results at different follow-up times, the most recent report published in a peer review was chosen. In case of incomplete or unclear data, authors were contacted. Disagreements were resolved by consensus.
QUALITY ASSESSMENT
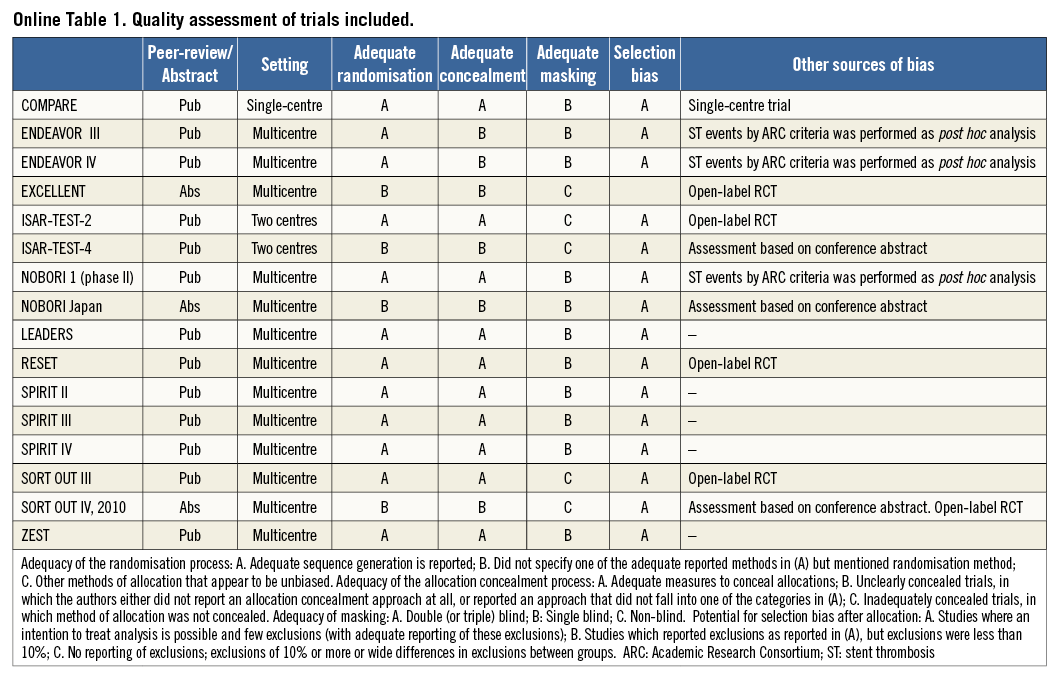
Two authors independently assessed the methodological quality of the included trials using a method proposed by the Cochrane Handbook for Systematic Reviews of Interventions4. In summary, quality assessment was performed using the following criteria: 1) peer review or abstract presentation; 2) setting; 3) adequacy of the randomisation process; 4) adequacy of the allocation concealment process; 5) potential for selection bias after allocation; 6) level of masking; and 7) presence of other sources of bias. Additional details regarding quality assessment are provided in Online Table 1.
DATA ANALYSIS
Inter-observer agreement for study selection and data abstraction was performed using Cohen’s weighted kappa. For each study, we calculated the summary risk ratios (RR) and their 95% confidence intervals for clinical outcomes. A random effects meta-analysis was used to estimate overall relative risks. Overall treatment effect was first calculated separately for RCTs comparing PES or SES versus second-generation DES and then for pooled data. In addition to recent direct comparisons between SES and EES, an adjusted indirect comparison between SES and EES based on pre-existing trials using a common comparator (PES) was performed according to the method laid out by Bucher et al7. In our study, data for the indirect comparison were obtained from a direct meta-analysis for the risk of ID-TLR, MI and death in trials comparing PES versus EES and data from a recently published meta-analysis of trials comparing SES versus PES8, to investigate the clinical benefit of EES versus SES. Since strut thickness, type of platform and polymer might affect clinical outcomes, a stratified analysis adjusted for these variables was performed. We also conducted sensitivity analyses to assess the effects of selected methodological and clinical variables on ID-TLR, MI, all deaths and ST.
We assessed the results for heterogeneity by examining the forest plots and then calculating a Q statistic which we compared with a χ2 distribution, and the I2 index. The potential sources of heterogeneity were investigated by stratified and meta-regression analysis. Variables included in the model were identified by ‡ in the Tables. Influence analysis for each outcome was performed. A study was considered influential if its exclusion changed the effect estimate by at least 20. The potential publication bias was assessed using funnel plots, Begg’s correlation and Egger’s regression. The p-value for heterogeneity (phet) was considered statistically significant when <0.10; otherwise, a p-value ≤0.05 was considered significant. Analyses were performed using Review Manager Version 5.1 (Cochrane Collaboration, Software Update, Oxford, UK) and Epidat version 3.1 (The EpiData Association, Odense, Denmark). The number needed to treat (NNT) and the number of avoided events per 1,000 treated patients were calculated as a marker of clinical relevance of using a given DES generation over the other, using Stata 12 statistical software (StataCorp LP, College Station, TX, USA).
Results
LITERATURE SEARCH
A description of the results of the RCTs search is shown as a flow chart in Figure 1. Our initial search yielded 152 references, of which 113 were excluded during the preliminary screening. Full texts of the remaining 38 articles were scrutinised to determine eligibility. Sixteen studies fulfilled eligibility criteria and were included for data abstraction. Six RCTs comparing PES versus second-generation DESs (ZES, EES or BES) and ten trials comparing SES versus ZES, EES or BES were found. The ZEST trial (Comparison of the Efficacy and Safety of Zotarolimus-Eluting Stent versus Sirolimus-Eluting and PacliTaxel-Eluting Stent for Coronary Lesions) performed a three-arm comparison of two G1 (PES and SES) and one G2 DES (ZES)9. By using the ZES arm as a common comparator, data from the ZEST trial were entered in our meta-analysis as separate comparisons of ZES against PES and SES, in accordance with the recommendations proposed by the Cochrane Handbook for Systematic Reviews of Interventions4. The ISAR-TEST-2 included three study arms: SES (CYPHER® stent), ZES and a polymer-free dual probucol-sirolimus DES10. Only data from the SES (G1 DES) and ZES (G2 DES) arms were entered in our meta-analysis. Likewise, the ISAR-TEST-4 included three treatment arms: biodegradable SES, non-erodible SES and non-erodible ESS11. Only data from non-erodible SES (G1 DES) versus non-erodible EES (G2 DES) arms were entered in this analysis. Agreement between investigators regarding data search was good (k=0.81).
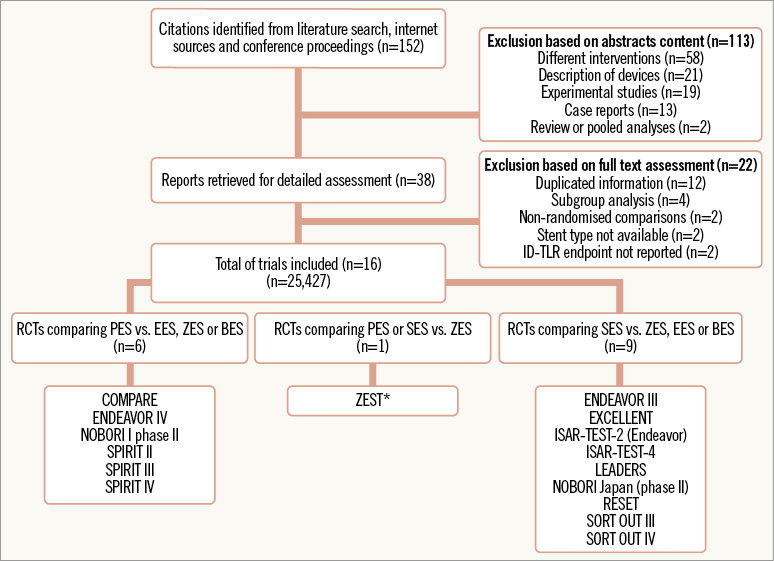
Figure 1. Study selection flowchart. *ZEST compared PES and SES versus ZES. BES: biolimus A9-eluting stent; EES: everolimus-eluting stent; ID-TLR: ischaemia-driven target lesion revascularization; PES: paclitaxel-eluting stent; SES: sirolimus-eluting stent; ZES zotarolimus-eluting stent
CHARACTERISTICS OF THE TRIALS INCLUDED
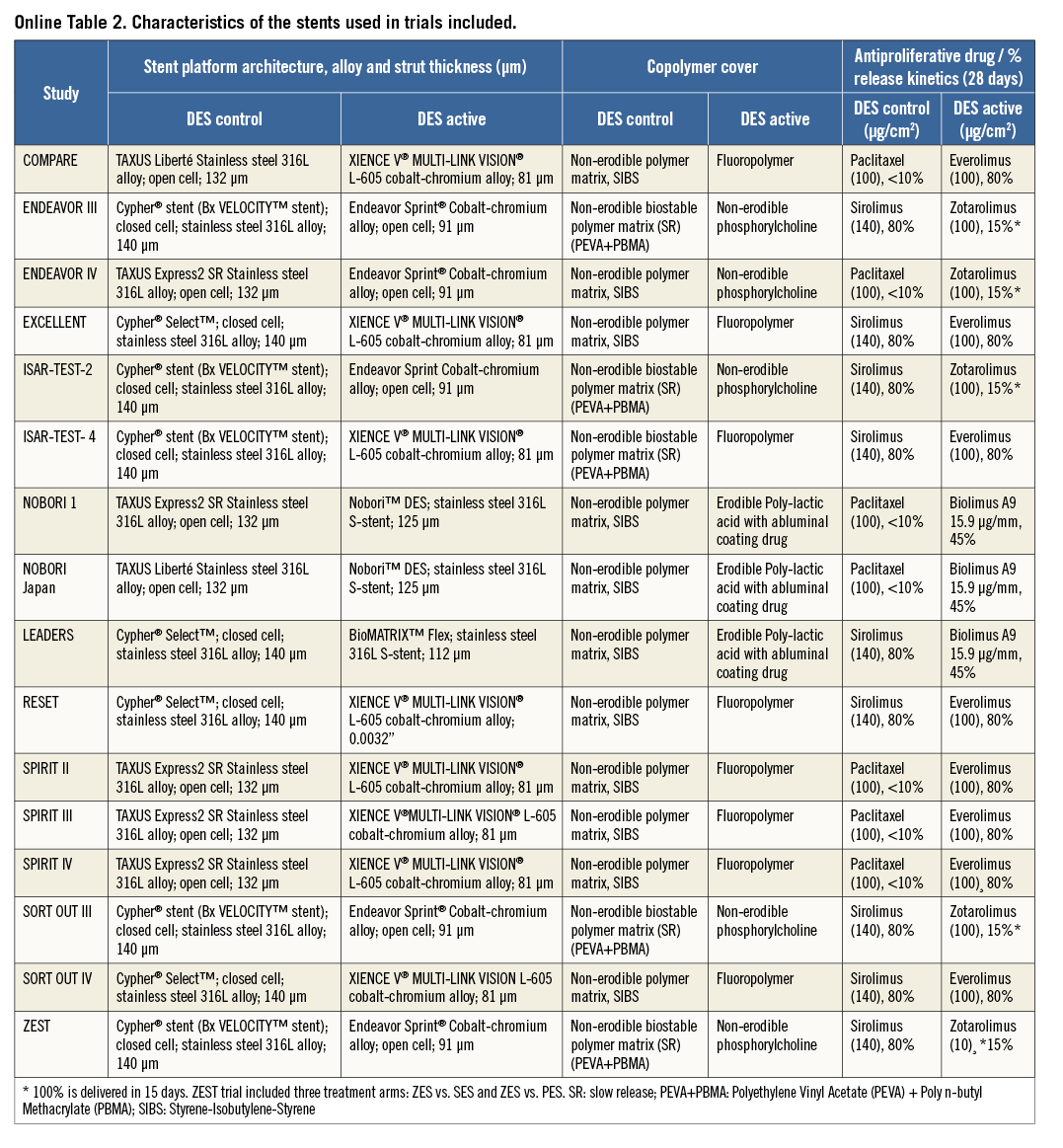
A total of 25,427 patients were included in the meta-analysis. More patients had been treated with G2 DES (13,653; 54%) than with G1 DES (11,774; 46%), because several studies used imbalanced randomisation12-15,17,21-23. Online Table 2 summarises relevant data on clinical and stent design variables for individual trials. Mean age ranged from 62 to 69 years, with the majority of patients being male. The frequency of diabetes mellitus ranged from 14 to 45% with no significant differences between compared groups except in the NOBORI trial 1 (phase 2), that included more diabetic patients in the G1 DES arm16. Follow-up ranged from nine to 60 months; the weighted mean follow-up was 21.8 months. Twelve trials had mandated angiographic follow-up, and the mean of angiographic follow-up was of 68%9-11,13-21. Duration of dual antiplatelet use in all trials ranged from three to 12 months.
The following RCTs followed a non-inferiority design: COMPARE, ENDEAVOR III, ENDEAVOR IV, EXCELLENT, ISAR-TEST-2, ISAR-TEST-4, NOBORI Japan, RESET, SPIRIT III and SORT OUT IV10-15,17,19,21,24. A non-inferiority/superiority design was followed in the NOBORI, LEADERS, SPIRIT II and SPIRIT IV RCTs17,18,20,22. Finally, a superiority approach was followed in the SORT OUT III only23. The ZEST trial compared a G2 DES (ZES) versus a G1 DES (SES) on a superiority design and a G1 DES (PES) on a non-inferiority design9.
Four trials (ENDEAVOR III, EXCELLENT, NOBORI 1 [phase 2], SPIRIT II) used angiographic primary endpoints (restenosis and late lumen loss)13,15,16,20, while the remaining trials used combined clinical endpoints as primary endpoints9-12,14,17-19,21-24. Four trials followed an all-comers design9,13,19,24. With the exception of the COMPARE trial, all studies were conducted in two or more centres.
Table 1 summarises the structural characteristics of DES platforms used in the included RCTs. Some of the structural changes introduced in G2 DES were the use of cobalt-chromium alloys, thinner strut thickness (≤95 microns) and erodible or biodegradable eluting polymers.
Direct comparisons between COMPARE, SPIRIT II, SPIRIT III and SPIRIT IV trials were performed to estimate the clinical benefit of EES over PES12,20-22. Likewise, data reported in a meta-analysis were used to estimate the performance of SES versus PES8. Then, adjusted indirect comparisons using a methodology proposed by Bucher were performed7.
RISK OF BIAS OF INCLUDED STUDIES
The majority of trials were published as peer-reviewed publications. The limited information on trial methodology in conference abstracts and presentations affected our ability to assess the quality of three trials15,17,24. The results of the quality assessment are presented in Online Table 1. Overall, no trials achieved “A” across all elements of the quality assessment. With regard to the adequacy of the randomisation process and concealment, a rating of “A” was given to 11 of the 16 trials9,10,12-14,16,18-23. The remaining trials were all rated as “B”. In relation to adequate masking, six trials were open-label and were therefore rated with a “C”10,11,15,19,23,24. The remaining trials were single-blinded; therefore, they were rated as “B”. The reporting of exclusions and the use of intention-to-treat analysis were very good across all trials: all were rated as “A”. Stent thrombosis analyses by ARC criteria were performed post hoc in three trials and these were considered as a potential source of bias13,14,16. An independent endpoint review committee participated in the adjudication of events across all trials.
ISCHAEMIA-DRIVEN TARGET LESION REVASCULARISATION
Figure 2 shows the forest plot of relative risk (RR) of ID-TLR in the included studies. Sixteen trials (17 comparisons) involving a total of 25,427 patients reported the incidence of ID-TLR in those allocated to G1 DES (n=11,774) or G2 DES (n=13,653) treatment. Pooled analysis revealed ID-TLR rates of 5% (1,289 events) for the overall population, 5.59% (647/11,774) for G1 and 4.70% (642/13,653) for G2 DES. No significant relative risk reduction (RRR) in ID-TLR was associated with the use of G1 or G2 DES (RR=0.91, 95% CI: 0.68-1.20, p=0.60). Significant heterogeneity was detected across studies, and meta-regression analysis identified length of follow-up (beta= -1.83, p=0.01) and stent type (beta= -0.58, p=0.05) as main determinants. Influence analysis yielded an RR range of 0.82 (95% CI: 0.62-1.11) to 0.95 (95% CI: 0.65-1.34), and exclusion of individual studies did not significantly modify the overall effect.
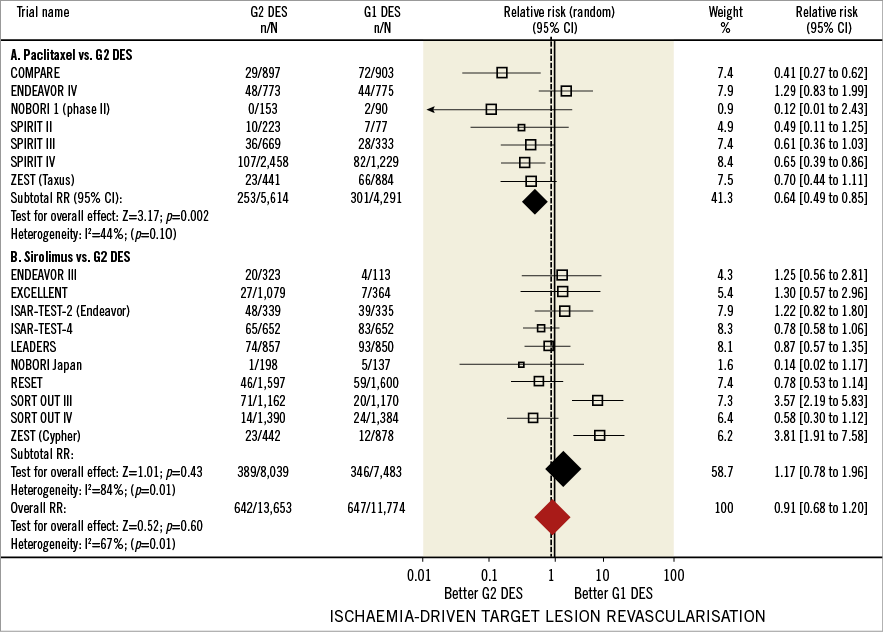
Figure 2. Ischaemia-driven target lesion revascularisation in first and second-generation drug-eluting stents. Forest plot illustrating the relative risk of ischaemia-driven target lesion revascularisation with first versus second-generation drug-eluting stents. Filled squares represent relative risks; square areas are proportional to the weight of individual studies; diamonds represent pooled relative risks; horizontal lines represent 95% confidence intervals.
Separate analysis of studies comparing G2 DES with PES disclosed ID-TLR rates of 6.57% for PES (282/4,291) and 4.39% for G2 DES (247/5,614), yielding a significant RRR of 36% in favour of G2 DES (RR=0.64, 95% CI: 0.49-0.85, p=0.002). For PES versus G2 DES comparisons, two studies reported statistically significant differences associated with G2 DES use12,22 while two studies favoured SES use when compared with G2 DES9,23 (Figure 2). Regarding the studies that compared G2 DES with SES, calculated ID-TLR rates were 4.62% for SES (346/7,483) and 4.83% for G2 DES (389/8,039). No significant RRR was found associated with the use of G2 DES or SES (RR=1.17, 95% CI: 0.98-1.16, p=0.43).
Adjusted indirect comparison analysis showed a significant RRR of 24% with EES use when compared with SES (RR=0.69, 95% CI: 0.56-0.85, p=0.05) (Online Figure 1A).
Stratified analysis of the included studies according to DES characteristics did not suggest that ID-TLR was influenced by stent strut thickness, erodible or non-erodible polymer (Figure 3).
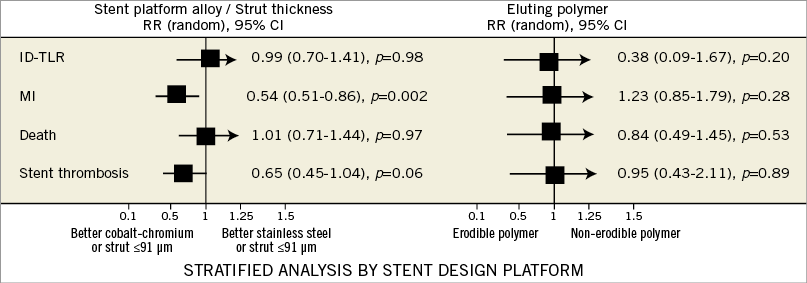
Figure 3. Stratified analysis of patient-focused outcomes according to study design characteristics and length of dual antiplatelet therapy. The Figure shows relative risks for patient-focused outcomes in a stratified analysis of pooled clinical trials according to inferiority or non-inferiority/superiority design, length of follow-up, performance of angiographic follow-up, and length of dual antiplatelet therapy (DAPT). * denotes a p-value ≤0.05. Boxes represent point estimates and lines 95% confidence intervals. G1 DES: first-generation drug-eluting stent; G2 DES: second-generation drug-eluting stent; ID-TLR: ischaemia-driven target lesion revascularisation; MI: myocardial infarction; RR: relative risk; ST: stent thrombosis
MYOCARDIAL INFARCTION
Figure 4 shows the forest plot of the relative risks of MI in all trials included. Myocardial infarction rates were reported in 15 studies (16 comparisons, 24,193 patients). The ISAR-TEST-4 was excluded from this analysis due to MI rates not being reported10. In total, 873 events (3.60%) were reported. In the overall population, the MI rate was significantly lower in G2 (2.97%, 387/13,001) than in G1 DES (4.36%, 486/11,122), with an associated 26% RRR (RR=0.74, 95% CI: 0.61-0.90, p=0.003). The number of MI events prevented by using G2 DES in 1,000 patients was 13.6 (95% CI: 9.2-17.5) (NNT=73.3). A significant heterogeneity was documented in the overall analysis (I2= 54%, phet=0.006), with length of follow-up and stent type (β=–0.63, p=0.04, β=–1,17, p=0.05, respectively) as main determinants. Influence analysis, which revealed RR shifts from 0.73 (95% CI: 0.52-0.94) to 0.75 (95% CI: 0.63-1.01), found no significant influence of individual studies on the overall effect.
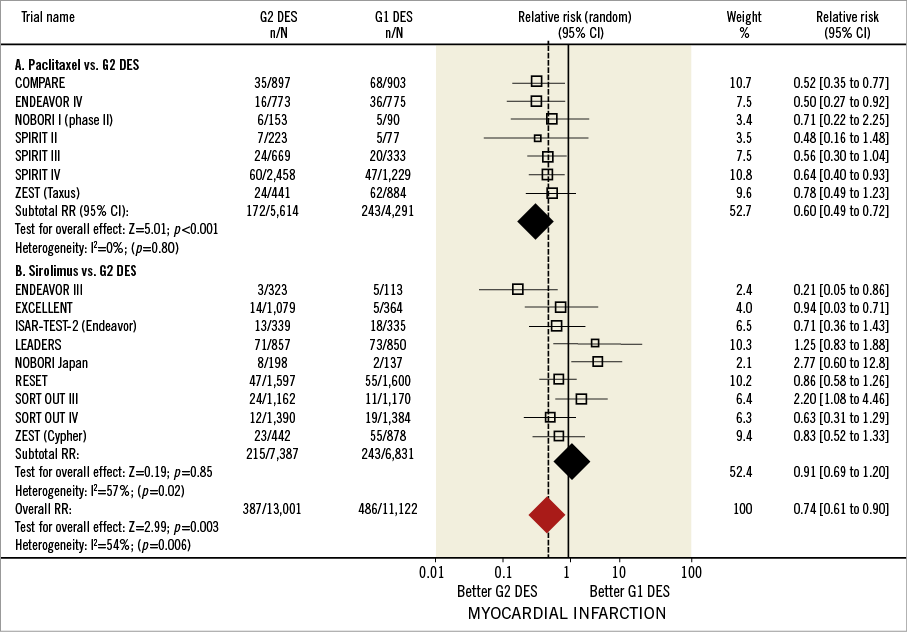
Figure 4. Myocardial infarction in first and second-generation drug-eluting stents. Forest plot illustrating relative risk of myocardial infarction with first versus second-generation drug-eluting stents. Filled squares represent relative risks; square areas are proportional to the weight of individual studies; diamonds represent pooled relative risks; horizontal lines represent 95% confidence intervals.
For the studies comparing G2 DES with PES, MI rates were 5.66% (243/4,291) for PES and 3.06% (172/5,614) for G2 DES, with a 40% RRR favouring G2 DES (RR=0.60, 95% CI: 0.49-0.72, p≤0.001). No significant heterogeneity was found in the studies comparing G2 with PES (I2=0%, phet=0.80). Conversely, no significant difference in MI rate was found between SES (3.55%, 243/6,831) and G2 DES (2.91%, 215/7,387) (RR=0.91, 95% CI: 0.69-1.20, p=0.85). Adjusted indirect comparison analysis revealed a significant RRR of 32% with EES use when compared with SES (RR=0.68, 95% CI: 0.50-0.93, p=0.01) (Online Figure 1B).
DEATH
Online Figure 2 shows forest plots for the endpoint of death. Thirteen studies (14 comparisons) reported the incidence of death in 22,018 patients (10,176 G1 and 11,842 G2 DES). The NOBORI Japan trial, SPIRIT II, and SORT OUT IV were not included since the all death rate was not reported17,20,24. No significant differences were found in death rates between G1 and G2 DES: 3.56% (363/10,176) in G1 and 3.79% (291/11,842) in G2 DES groups (RR=0.89, 95% CI: 0.74-1.05, p=0.17). No heterogeneity among studies was detected (I2=27%, phet=0.17), and sensitivity analysis did not reveal significant changes in the overall effect with exclusion of individual studies (RR shift from 0.92 [95% CI: 0.73-1.10] to 1.04 [95% CI: 0.82-1.23]). Only one trial showed a significant reduction of death risk with the use of G2 DES13 (Figure 5).
In the studies comparing PES with G2 DES, death rates were 2.82% (119/4,214) for PES and 2.42% (131/5,391) for G2 DES. This difference was non-significant (RR=0.83, 95% CI: 0.65-1.06, p=0.14). In studies comparing SES with G2 DES there were no significant differences: 3.23% (141/4,362) for SES and 3.31% (161/4,854) for G2 DES; the overall effect was comparable between both groups (RR=0.93, 95% CI: 0.66-1.32, p=0.14). As with previous endpoints, an adjusted indirect comparison analysis was performed to compare SES with EES, but non-significant RRR associated with EES use was found (RR=0.98, 95% CI: 0.82-1.18, p=0.86) (Online Figure 1C).
STENT THROMBOSIS
Figure 5 shows the forest plot for ST (definite and probable stent thrombosis according to ARC criteria). The incidence of ST was reported in 16 studies (17 comparisons) involving a total of 25,427 patients. The overall prevalence of ST was 1.10% (282 events). There were no significant overall differences in ST prevalence in G1 and G2 DES: 1.35% (159/11,774) in the G1 DES and 0.90% (123 of 13,653) in the G2 DES (RR=0.69, 95% CI: 0.54-0.88, p=0.004). Per G1 DES comparator, ST rates were 1.84% and 1.07% for TAXUS and CYPHER, respectively.
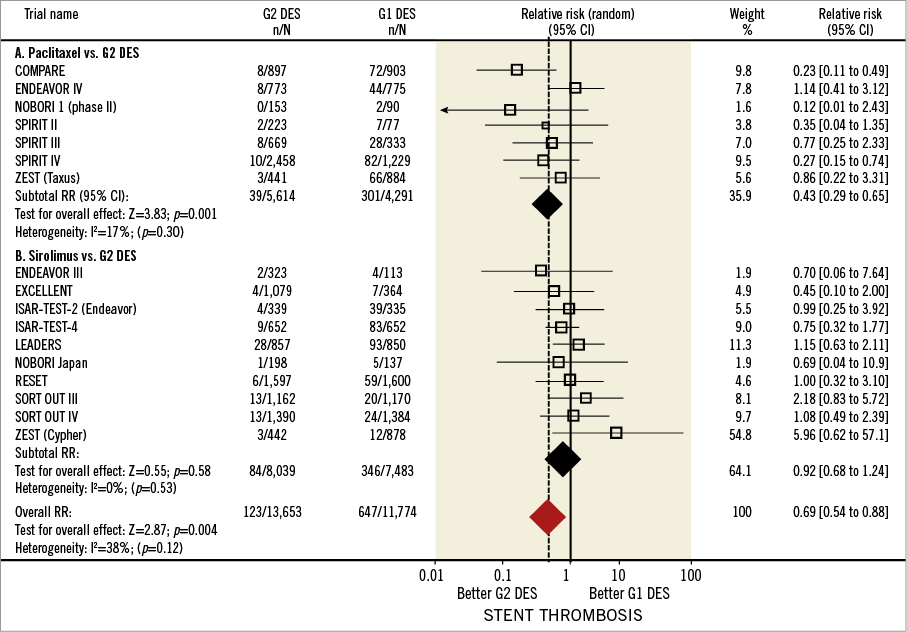
Figure 5. Stent thrombosis in first and second-generation drug-eluting stents. Forest plot illustrating the relative risk of stent thrombosis with first versus second-generation drug-eluting stents. Filled squares represent relative risks; square areas are proportional to the weight of individual studies; diamonds represent pooled relative risks; horizontal lines represent 95% confidence intervals.
Separate analysis of the studies comparing PES and G2 DES revealed a significant RRR (59%) associated with G2 DES use (RR=0.41, 95% CI: 0.26-0.65, p<0.01). The number of ST events prevented by using G2 DES in 1,000 patients was 4.2 (95% CI: 1.7-6.1) (NNT=239.7). On the contrary, no significant differences in ST were found in the comparison between SES and G2 DES (RR=0.92, 95% CI: 0.68-1.24, p=0.58). There was no evidence of significant heterogeneity among studies (I2=38%, phet=0.12). Sensitivity analysis revealed an RR ranging from 0.69 (95% CI: 0.41-1.08) to 0.89 (95% CI: 0.57-1.38), showing no significant influence of individual studies on the overall effect.
SENSITIVITY ANALYSIS ACCORDING TO METHODOLOGICAL AND CLINICAL VARIABLES
We evaluated the consistency of our analyses by performing stratified analyses for ID-TLR, MI, death and ST by selected methodological and clinical variables (Figure 6). For ID-TLR and death, the overall treatment effect of G2 DES remained consistent for each variable using either design, length of follow-up and clopidogrel duration. However, the ID-TLR treatment effect varied positively for G2 DES in trials with protocol angiographic follow-up (RR=0.82, 95% CI: 0.62-1.00, p=0.05). For MI, the overall benefit observed for G2 DES was consistent in all the variables explored. Nevertheless, regarding clopidogrel duration, the benefit was observed in those trials when the duration of dual antiplatelet therapy was extended until 12 months9,12,14,18,23,24. A significantly lower relative risk of MI (RR=0.54, 95% CI: 0.51-0.86, p=0.002) was associated with the use of G2 DES with thin struts (<91 microns). No effect of erodible or non-erodible polymer was documented (Figure 6).
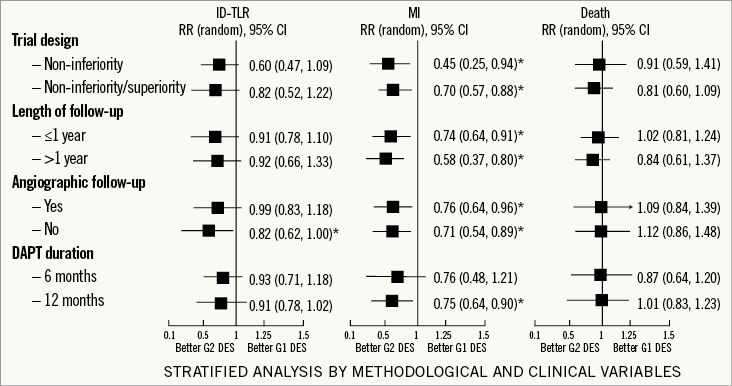
Figure 6. Stratified analysis of patient-focused outcomes according to stent strut thickness and type of eluting polymer. The Figure shows relative risks for patient-focused outcomes in a stratified analysis of pooled clinical trials according to stent strut thickness and type of eluting polymer. The pooled estimates are reported as relative risk. Boxes represent point estimates and lines 95% confidence intervals. ID-TLR: ischaemia-driven target lesion revascularisation; MI: myocardial infarction; RR: relative risk; ST: stent thrombosis
PUBLICATIONS BIAS
Visual inspection of funnel plots did not reveal asymmetry for the endpoints MI, death and ST. In support, the Begg’s rank correlation and Egger’s regression test were not statistically significant. Although some asymmetry was noted in the funnel plot for endpoint ID-TLR, the existence of publication bias for this endpoint was ruled out by the non-significant results of the Begg’s rank correlation and Egger’s regression test (p=0.84 and p=0.84, respectively). Similar results were obtained for MI, death and ST. Finally, the majority of studies were classified as low risk of bias across all domains of study quality (Online Table 1).
Discussion
The systematic review and trial level meta-analysis reveal that technological modifications introduced in G2 DES have translated into better patient outcomes than documented with G1 DES. Since the study revealed the existence of major sources of bias in the use of a G1 comparator, a separate analysis was performed for PES and SES as comparators. Overall, changes introduced in G2 DES technology have clearly increased the level of safety and efficacy reached with G1 DES. Although direct pooled analysis of available RCTs comparing G1 versus G2 DES did not show a clear clinical benefit in terms of ID-TLR risk reduction, a statistically significant rate of reduction of MI and stent thrombosis favouring G2 DES was documented in our analysis. Moreover, an indirect comparison of existing trials revealed a superiority of EES over SES in terms of ID-TLR. Importantly, the use of thinner stent struts was identified as a contributor to better outcome of G2 DES. In the following paragraphs these issues are discussed in detail.
The pace of developments in the field of percutaneous revascularisation implies an almost constant lag between evidence and use, or even availability, of tested devices. Most of the available evidence on the safety and efficacy of DES is based on the evidence gathered with the CYPHER and TAXUS DES in major trial programmes; however, they will soon be no longer available (TAXUS Liberté has been replaced by the thinner strut, platinum chromium alloy PES TAXUS Element, and the production of CYPHER Select was discontinued by the end of 2011)25,26. As a consequence of this, percutaneous revascularisation will be carried out using G2 DES that have been approved by regulatory authorities on the grounds of trials comparing G1 and G2 DES. Many of these trials followed a non-inferiority or sequential non-inferiority/superiority design for the primary outcome, which frequently was a composite endpoint. The aim of this study was to investigate whether the modifications introduced in the structural and antiproliferative components of G2 DES with the aims of improving safety and efficacy have translated to better patient-oriented outcomes. For this reason, combined endpoints were avoided, and individual outcomes (death, MI and ID-TLR) were used instead27. Studies not reporting patient-oriented outcomes could not, therefore, be included in our analysis.
THE EFFECT OF G1 COMPARATOR ON ASSESSMENT OF G2 DES PERFORMANCE
Since the study revealed the existence of major sources of bias in the use of a G1 comparator, a separate analysis was performed for PES and SES as comparators, and major differences in the outcome of G2 DES when compared with the TAXUS or CYPHER G1 DES were found. The choice of a comparator constitutes one of the key issues in designing valid non-inferiority/equivalency trials28. In spite of the fact that the TAXUS stent was introduced after the G1 CYPHER stent, much of the available evidence on G2 DES comparisons has been obtained in patients included in trials that used TAXUS as a G1 comparator (41% of overall included patients). The preference for the use of the TAXUS stent as a comparator in non-inferiority trials was not specified in any study.
Regarding the G1 TAXUS stent, our analysis demonstrates that its efficacy and safety have been improved by the modifications made in G2 DES, as judged by the pooled analysis of comparative studies with EES, ZES and BES. A consistent improvement, supported by a lack of heterogeneity between studies, was observed in terms of reduction of ID-TLR, MI and stent thrombosis (Figure 2, Figure 5 and Online Figure 2).
Although at first glance the results of our analysis do not suggest that G2 have improved outcome when compared with G1 Cypher, attention should be paid to the important bias in comparisons with G2 EES before drawing conclusions. Although direct pooled comparisons of the CYPHER stent versus G2 DES reveal no significant improvement regarding safety and efficacy, removing ZES comparison results (ENDEAVOR III, ISAR-TEST-2, SORT OUT III and ZEST [CYPHER]) from analysis revealed a statistically significant benefit in terms of ID-TLR and MI favouring G2 DES (RR=0.78, 95% CI: 0.65-0.92, p=0.02) and (RR=0.81, 95% CI: 0.73-0.98, p=0.04), respectively (data not included in forest plot). This finding implies that heterogeneities between different G2 DES should be taken into account in interpreting these results. On the other hand, RCTs comparing EES account for 68% of all patients (6,789/9,905) included in trials using TAXUS as a G1 comparator, but only for 45% (5,521/12,235) of patients included in RCTs with CYPHER as comparator. These differences in the selection of the G1 DES comparator could constitute a source of bias, and might potentially have influenced our analysis.
We also performed an indirect comparison between G2 EES versus G1 DES, based on the following reasons: 1) presence of conflicting results in individual trials comparing SES versus EES; 2) need to exclude several studies comparing SES and EES on the grounds that ischaemia-driven TLR, a primary efficacy endpoint in our meta-analysis, was not reported; and 3) need to provide as much evidence as possible on the effect of G2 DES on patient outcomes, compared with G1, in a research scenario that has prompted the use of PES as a comparator against G2 DES. Indirect comparison meta-analyses have recently been proposed and been shown to predict rather reliable results of subsequent direct randomised comparisons7.
The results of this indirect comparison suggest, compared to the CYPHER stent, a distinct benefit of G2 EES for the endpoints of ID-TLR and MI. Some of these results are supported by the LESSON-1 study, a historically controlled, non-randomised study comparing EES and SES28. In LESSON-1, the decrease in MI associated with G2 EES (hazard ratio [HR] 0.62, p=0.017) was virtually identical to that estimated in our indirect comparison (RR 0.68, p=0.01), as well as the risk of death (HR 0.88 in LESSON-1 [p=0.59], RR 0.96 in our study [p=0.92]). Conversely, no significant differences in TLR in LESSON-1 (HR 0.80, p=0.80) were found between EES and SES, while in our indirect comparison a statistically borderline difference favouring EES was noted (RR 0.76, p=0.05). It is not possible to infer from our trial-level meta-analysis whether differences in TLR were related to stent thrombosis. In LESSON-1, differences in target vessel revascularisation (TVR) between SES and EES were partially related to a lower prevalence of ST in EES-treated patients28.
INFLUENCE OF THE DESIGN OF RCTS
By combining data from multiple trials in a meta-analysis we sought to overcome the limitations of non-inferiority margin election and other methodological problems that have been attributed to non-inferiority designs28. We also performed a separate sensitivity analysis to investigate whether the conclusions of RCTs with a non-inferiority design were different from those with non-inferiority/superiority design, without finding significant differences.
Conversely, in the RCTs with angiographic follow-up no significant advantage of G2 DES over G1 DES could be demonstrated regarding the endpoint of ID-TLR. The G1 comparators in trials with angiographic follow-up were the TAXUS stent in four RCTs9,16,20,21 and the CYPHER stent in eight RCT9-11,13-15,17,18. The former finding can be explained by the well-known increase in TLR triggered by angiographic follow-up29.
INFLUENCE OF STRUCTURAL CHARACTERISTICS OF DES
We also investigated the impact on patient outcome of specific changes introduced in G2 stent platforms to improve stent haemorrheology and biocompatibility, such as decreased strut thickness and biodegradable eluting matrices.
Stratified analysis of pooled RCTs revealed that the use of thin-strut DES design is associated with a significant reduction of MI. Again, a bias in G1 comparator was noted in the use of thin-strut G2 DES. Cobalt-chromium G2 DES with <0.91 µm strut thickness were used in 68% (6,789/9,905) of patients included in RCTs with PES as comparator, but only in 45% (5,521/12,235) of those comparing G2 and SES, that was more frequently compared with stainless steel 119 µm thick-strut DES (Nobori and Biomatrix) than the TAXUS stent. Thus, the preponderance of thin-strut G2 DES in comparisons with PES may also have contributed to the significantly higher reduction of MI observed in G2 in those trials. The finding that DES strut thickness influences outcome is supported by previous studies reporting a proportional relationship between stent strut thickness and thrombogenicity30, strut malapposition31, and decreased neointimal coverage32. Overlapping of DES, which is frequently required to treat tapered, long or bifurcation stenoses, has been identified in clinical series as a predictor of MI33. At these overlapping locations, haemodynamic phenomena, increased flow separation, stagnation and reattachment are magnified by strut thickness34.
Non-erodible polymer matrices have been proposed as a cause of persistent inflammatory vascular reactions which might be causative in long-term appearing stent malapposition, incomplete strut coverage and stent thrombosis35. Three of the trials included in our analysis tested a G2 DES with a polylactic acid biodegradable-eluting matrix in a relatively small number of patients (10% of G2 DES, 1,208/12,056), more frequently against a PES G1 comparator (87%, 1,055/1,208). No significant advantage of this particular feature could be detected in the sensitivity analysis performed. A recent study by Navarese et al which focused on this topic also failed to detect significant advantages in target lesion revascularisation, MI and death, of biodegradable polymer DES compared with non-erodible polymer designs36.
INFLUENCE OF G2 DES TECHNOLOGY ON STENT THROMBOSIS
Our analysis revealed a statistically significant reduction in the risk of stent thrombosis associated with the use of G2 DES (RR=0.69, 95% CI: 0.54-0.88, p=0.004). Moreover, a post hoc trial analysis including studies reporting very late ST rates showed a statistically significant benefit of G2 DES (0.96%; 47/4,873) over G1 DES (1.72%; 71/4,115), (RR=0.62, 95% CI: 0.42-0.92, p=0.02)10-14,16-20. Although this finding could be influenced by comparisons of G2 DES versus PES, some changes introduced into newer design features in G2 DES may account for these results. For instance, flow disturbances caused by strut thickness may affect endothelialisation which is minimised by G2 DES improvements37. Likewise, drug concentration changes introduced in G2 DES may potentially reduce the toxicity and local inflammation, which both improve healing38. Finally, the use of erodible polymers in newer DES has been recognised as an important contributor to lower ST risk. This was proven by a recent meta-analysis comparing the safety and efficacy of biodegradable versus durable polymer DES which showed a significant reduction of late/very late ST rate (RR=0.60, 95% CI: 0.39-0.91, p=0.02)36.
Limitations
There are additional limitations of our study to those mentioned in the above discussion. Our meta-analysis was not performed at a patient level, and therefore the effect of study population characteristics could not be addressed. Although in most analyses no statistically significant heterogeneity was detected, it is quite clear that other sources of heterogeneity among studies exist, including clinical settings, stent platforms and eluting polymers. Relevant issues such as drug-elution kinetics could not be addressed. Intracoronary guidance of DES implantation was not included as part of the study protocol in any of the trials. Assessment of ST was hampered by the lack of: 1) details on when ST occurred (cumulative incidence of ST could not therefore be calculated); and 2) data on clopidogrel adherence.
Conflict of interest statement
The authors have no conflicts of interest to declare.
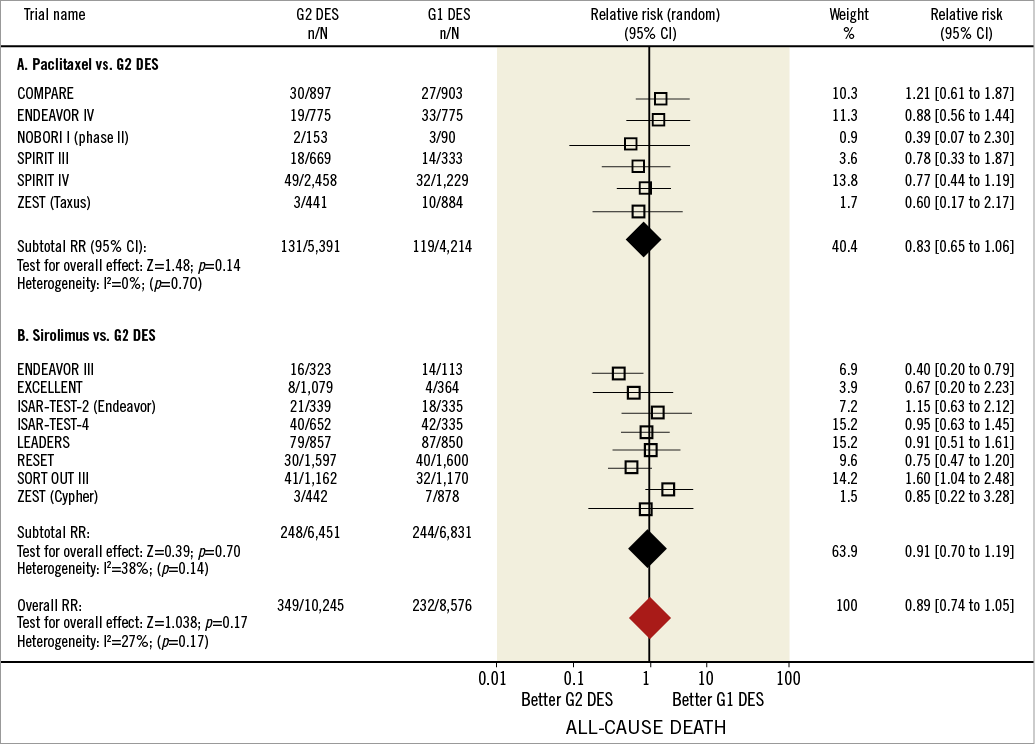
Online Figure 1. Death in first and second-generation drug-eluting stents. Forest plot illustrating the relative risk of all deaths with first versus second-generation drug-eluting stents. Filled squares represent relative risks; square areas are proportional to the weight of individual studies; diamonds represent pooled relative risks; horizontal lines represent 95% confidence intervals.
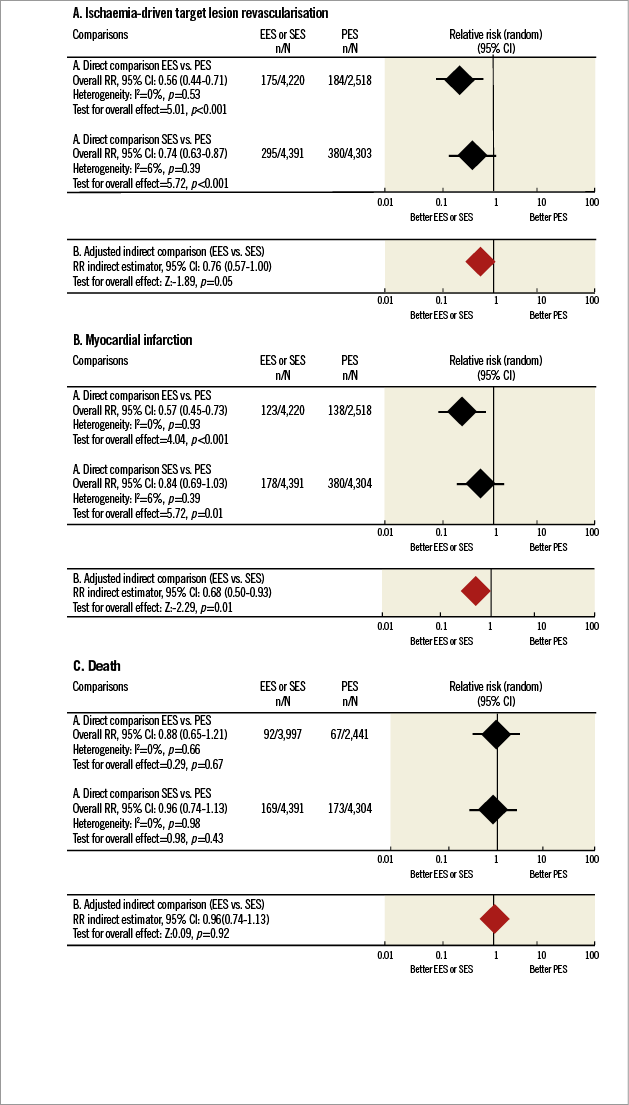
Online Figure 2. Indirect comparisons between sirolimus-eluting stents and everolimus-eluting stents based on randomised clinical trials that use paclitaxel-eluting stents as a common comparator. The Figure shows the result of adjusted indirect comparisons (lower panels) between sirolimus (SES) and everolimus (EES)-eluting stents based on trials comparing SES and paclitaxel-eluting stents (PES) and trials comparing EES and PES (upper panels). PES serve as a common comparator in these indirect comparisons. Indirect comparisons are shown for the following endpoints: A) ischaemia-driven target lesion revascularisation; B) myocardial infarction; and C) death. Black diamond represents pooled relative risks for direct comparison while red diamond shows the benefit for the adjusted indirect comparison. Horizontal lines represent 95% confidence intervals. EES: everolimus-eluting stent; PES: paclitaxel-eluting stent; RR: relative risk; SES: sirolimus-eluting stent