Abstract
Aims: The role of drug-eluting stent (DES) remains an unsettled issue in patients with ST-segment elevation myocardial infarction (STEMI). Therefore, we performed a meta-analysis of randomised trials to evaluate the clinical outcome of DES as compared with bare-metal stent (BMS) after percutaneous coronary intervention (PCI).
Methods and results: We undertook a literature search until July 2009. Thirteen clinical trials met inclusion criteria, with 7,244 patients enrolled. Up to 1-year, patients treated with DES as compared with BMS experienced less target-vessel revascularisation (TVR) (5.11% versus 11.19% respectively, p<0.00001) and recurrent myocardial infarction rates (3.03% versus 3.70% respectively, p=0.02). In addition, no significant differences were found in terms of cardiac death (2.80% versus 3.52%, p=0.21) and stent thrombosis (2.65% versus 2.76%, p=0.37).
Using the adjusted indirect comparison, a significant difference between sirolimus- and paclitaxel-eluting stent was found when TVR was evaluated (OR [95% CI] =0.59 [0.40-0.89], p=0.01), without differences in other clinical outcomes.
Conclusions: In patients undergoing PCI for STEMI, treatment with DES is associated with decreased TVR and myocardial infarction rates, without increasing cardiac death or stent thrombosis occurrence. Sirolimus-eluting stent is associated with a greater TVR reduction as compared to paclitaxel-eluting stent.
Introduction
Despite improvements in therapeutic options for patients with ST-segment elevation myocardial infarction (STEMI),1 approximately one of 10 patients treated still dies.2 When feasible, primary percutaneous coronary intervention (PCI) is the optimal reperfusion therapy for STEMI patients,3 leading to a significant reduction in mortality rates when compared to pharmacologic therapy alone.4 Moreover, in this setting, it has been demonstrated that a strategy of primary PCI with bare-metal stents (BMS), when compared to only balloon angioplasty, further improves the outcome, reducing the need for repeat revascularisation without any impact on mortality.5 Therefore, while BMS implantation has become the dominant therapeutic option for primary PCI, the role of drug-eluting stents (DES) is less well established, and treatment of STEMI patients still remains an “off-label” indication for these devices. However, since DES reduce the risk of reintervention when compared with BMS in elective PCI, these devices might have the potential to further improve clinical outcome after primary PCI.6 Several randomised controlled trials (RCTs) have examined the clinical outcome of the two most commonly used DES –sirolimus-eluting stent (SES) and paclitaxel-eluting stent (PES)– in patients with STEMI. Meta-analysis of RCTs has the potential to increase power and summarise results from different, but comparable, individual studies.7 Therefore, we performed a meta-analysis of RCTs comparing clinical outcome of DES with BMS in STEMI patients up to the 12-month follow-up.
Methods
Search strategy and selection criteria
We searched Medline, EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL), scientific session abstracts in Circulation, the Journal of the American College of Cardiology, the European Heart Journal and The American Journal of Cardiology, and relevant websites (www.acc.org, www.americanheart.org, www.europcr.com, www.escardio.org, www.clinicaltrialresults.org, www.tctmd.com and www.theheart.org) for studies in any language (from inception of each database until July 2009). The reference list of relevant studies was additionally scanned. The key words used were: “randomised trial”, “myocardial infarction”, “ST-segment elevation”, “percutaneous coronary intervention”, “angioplasty”, “primary angioplasty”, “drug eluting stent”, “sirolimus eluting stent”, “paclitaxel eluting stent”, “bare metal stent”. To be included, the citation had to meet the following criteria: 1) random treatment allocation and 2) a mean follow-up period ≥3 months. Exclusion criteria were: 1) ongoing studies and 2) irretrievable data.
Data collection and quality assessment
Two investigators independently assessed reports for eligibility at title and/or at abstract level, with divergences resolved with a third reviewer; studies that met inclusion criteria were selected for further analysis. Studies were evaluated with respect to the following methodological items: randomisation, adequacy of allocation concealment, performance of the analysis according to the intention-to-treat principle, sample size calculation and specification of loss of patients at follow-up. We did not use a quality score, since this practice has been previously discouraged.8
Outcome variables
The primary efficacy endpoint was target-vessel revascularisation (TVR), and the primary safety endpoint was stent thrombosis (ST). Secondary endpoints were cardiac death and recurrent myocardial infarction (MI). All clinical endpoints were evaluated according to per protocol definitions, up to 1-year follow-up.
Statistical analysis
The κ statistic was used to assess agreement between reviewers for study selection. Odds ratio (OR) and 95% confidence intervals (95% CI) were used as summary statistics and the pooled OR was calculated by using a fixed-effect model with the Mantel-Hænzel method. The Breslow-Day chi-squared test was calculated to test the statistical evidence of heterogeneity across the studies (p <0.1). In addition, we used the I2 statistic, which describes the percentage variation across studies that is due to heterogeneity rather than chance.9 The DerSimonian and Laird random-effect model was used to calculate the pooled OR in case of significant heterogeneity across studies. A funnel plot as well as the adjusted rank correlation test, according to the method of Begg and Mazumdar,10 were used to assess publication bias with respect to each endpoint. Moreover, we performed a sensitivity analysis, in which the meta-analysis estimates are computed omitting one study at time. To test the robustness of results in case of fixed-effect model application, significant results were evaluated with random-effect model too. We then used the test of interaction11 to examine whether clinical endpoints were influenced by dual antiplatelet therapy discontinuation (RCTs that interrupted thienopyridine versus RCTs that continued thienopyridine at the time of follow-up), state of publication (full-length articles versus not yet published articles), sample size (trials with ≥300 patients versus <300 patients), enrolling centers (multicentre versus single-centre trials) and follow-up length (trials with 1-year follow-up versus trials with <1-year follow-up). To rule out a possible influence of angiographic follow-up and “oculostenotic reflex” on TVR incidence, the interaction between routine angiographic follow-up and TVR was also provided. Association between the baseline risk of TVR, measured as the incidence of TVR among patients allocated to controls (i.e., BMS), and the clinical benefits from DES implantation instead of BMS, i.e., the number of patients needed to be treated (NNT) to prevent one TVR, was investigated by a weighted least-square regression (WLS), using as WLS variable the number of patients included in each study. Finally, we undertook an adjusted indirect comparison12 to investigate differences between SES and PES, assuming BMS arms as common comparator. In accordance with the Cochrane protocol13, we generated, from random effect OR comparing SES versus BMS and PES versus BMS, an interaction OR with pertinent 95% CI and z scores for two-tailed hypothesis testing (p is significant if <0.05). Statistical analyses were performed with Review Manager 5.0.16 RevMan [Computer program]. Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008, SPSS 16.0 statistical package (SPSS Inc., Chicago, Illinois, USA) and Stata 10.0 statistical software (STATA Corp, College Station, Texas, USA).
The study was realised in compliance with the Quality of Reporting Meta-analyses (QUORUM) Guidelines.14
Results
Eligible studies
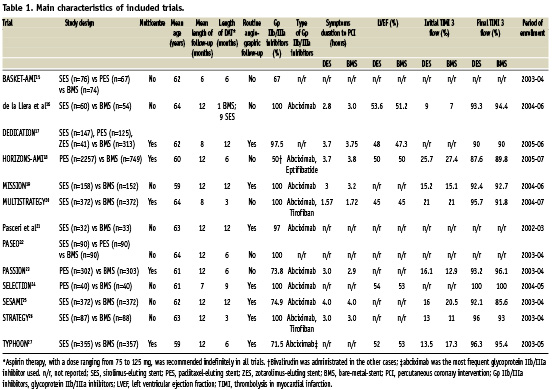
We screened the title or the abstract of 959 potentially eligible publications as well as 13 randomised trials which were included in the meta-analysis,15-27 enrolling 7,244 patients (4,459 or 61.55% randomised to DES and 2,785 or 38.45% randomised to BMS) (Figure 1). The inter-observer agreement for study selection was very good, with κ=0.98. SES was used in seven trials16,19-21,25-27 and PES in three trials;18,23,24 in the remaining three trials both SES and PES were included,15,17,22 with zotarolimus-eluting stent implanted in about 13% of one study.17 Main characteristics of included trials were reported in Table 1. Stent thrombosis was defined as an acute coronary syndrome with angiographically documented thrombus within stent in all trials. In eight trials this definition was expanded to include patients with unexplained sudden death16-18,20,21,25,27 or patients who had Q-wave MI in the territory of the stented vessel.16-21,25,27 A clopidogrel loading dose of 300 to 600 mg was administered before PCI in all trials included. Nine RCTs enclosed in this meta-analysis provided detailed description of appropriate randomisation methods, mainly based on computer-generated randomisation lists.16-20,23,25-27 Seven out of 13 study reports17-20,23,26,27 described the use of concealed allocation method, all but two using sealed envelopes; all studies reported the number of patients, if any, lost to follow-up. Nine studies reported sample-size calculations.17-20,23-27 The analysis according to the intention-to-treat principle was performed in all trials, and, in two of them, a modified intention-to-treat principle, comprising of exclusion of patients who did not receive the study stent, was used.19,27
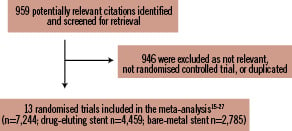
Figure 1. Flow diagram of trial selection.
Clinical endpoints
TVR occurred in a total of 542 patients (7.48%). As reported in Figure 2, treatment with DES was associated with a significant TVR reduction (5.11% vs 11.27%, DES vs BMS respectively, OR [95% CI] =0.43 [0.35-0.51], p<0.00001), NNT=17 (15-20, 95% CI). There was a moderate heterogeneity across trials (I2=33%, p=0.12). However, neither eight funnel plot nor rank correlation test (p>0.15) pointed out any publication bias. In addition, we found a significant relationship between the incidence of TVR in patients allocated to BMS in each trial and the NNT for TVR in each of these trials (β: –0.73 [95% CI: –3.77; –0.91], p=0.004) (Figure 3).
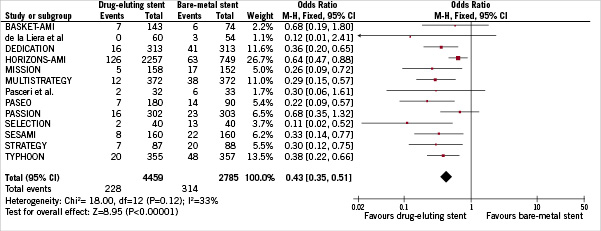
Figure 2: Odds ratio of target-vessel revascularisation associated with drug-eluting stent versus bare-metal stent. The squares and the horizontal lines indicate the OR and the 95% CIs for each trial included; the size of each square is proportional to the statistical weight of a trial in the meta-analysis; diamond indicates the effect estimate derived from meta-analysis, with the center indicating the point estimate and the left and the right ends the 95% CIs.
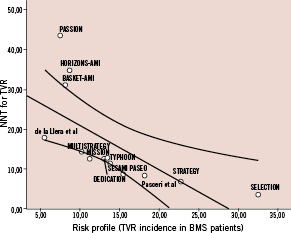
Figure 3: Regression analysis. The number-needed-to treat (NNT) for target-vessel revascularisation (TVR) in each trial was plotted against the risk of clinical restenosis among the control group (assumed as TVR incidence in patients allocated to bare-metal stent treatment). A significant relationship was found between these two variables.
Figure 4 shows that ST was experienced from a total of 195 patients (2.69%) up to 1-year. The cumulative incidence of acute, subacute and late stent thrombosis was similar between DES and BMS, without significant difference among two groups (2.65% vs 2.76% respectively, OR [95% CI] =0.87 [0.65-1.18], p =0.37). There was no heterogeneity across trials (I2=0%). No evidence of publication bias was found using funnel plot and rank correlation test (p =0.35).
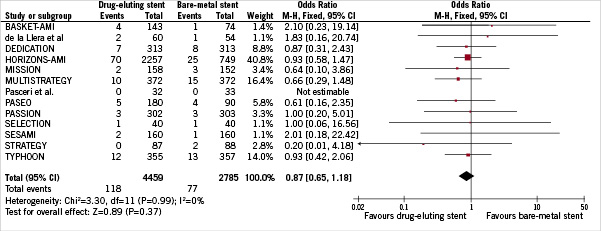
Figure 4. Odds ratio of stent thrombosis associated with drug-eluting stent versus bare-metal stent.
Cardiac death was observed in a total of 223 patients (3.08%). As reported in Figure 5, the overall incidence of cardiac mortality was not different between DES and BMS cohorts (2.80% vs 3.52%, DES vs BMS respectively, OR [95% CI] =0.84 [0.63-1.10], p=0.21). No evidence of heterogeneity was noted across trials (I2=0%). No evidence of publication bias was found using funnel plot and rank correlation test (p>0.63).
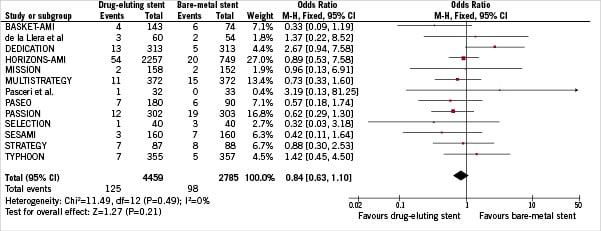
Figure 5. Odds ratio of cardiac death associated with drug-eluting stent versus bare-metal stent.
A total of 238 patients (3.28%) experienced recurrent MI. As depicted in Figure 6, a significant difference in terms of recurrent MI was found in favour of DES (3.03% vs 3.70% DES versus BMS respectively, OR [95% CI] =0.73 [0.56-0.96], p=0.02). There was no heterogeneity across trials (I2=0%). Both the funnel plot and rank correlation test (p>0.43) did not disclose any publication bias.
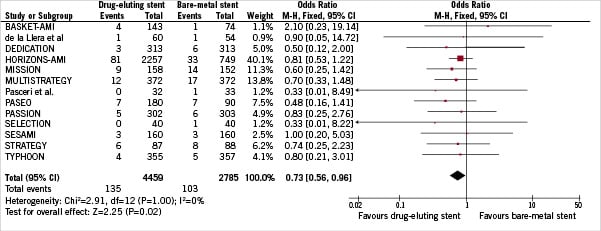
Figure 6. Odds ratio of recurrent myocardial infarction associated with drug-eluting stent versus bare-metal stent.
Sensitivity and subgroup analyses
Sensitivity analysis demonstrated that no single study significantly altered the summary ORs, since one at a time study omission did not result in a movement of the point estimate outside the 95% CIs. Also with random effects, ORs remained in favour of DES for TVR (0.39 [95% CI, 0.30-0.51], p <0.0001) and recurrent MI (0.73 [95% CI, 0.56-0.96], p=0.03). As reported in Table 2, discontinuation of dual antiplatelet therapy at follow-up, the status of publication, sample size, enrolling centers number and mean length of follow-up did not affected results, with the exception of trials with small sample size (<300 patients) that found a stronger TVR reduction as compared with large trials (≥300 patients) (OR=0.26 [95% CI, 0.16-0.43] versus OR=0.47 [95% CI, 0.38-0.57], p-interaction =0.03). In addition, TVR reduction associated with DES was greater in trials performing routine angiographic follow-up as compared to trials without angiographic follow-up (OR=0.32 [95% CI, 0.24-0.44] vs OR=0.52 [95% CI, 0.41-0.66] respectively, p-interaction =0.01).
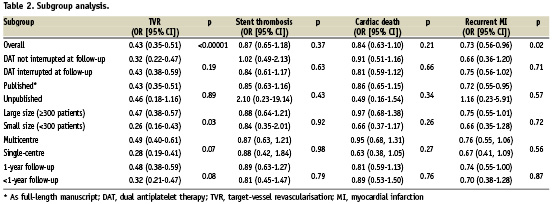
Adjusted indirect comparison of SES versus PES
At the indirect comparison, a significant difference between SES and PES was found when TVR was evaluated (OR [95% CI] =0.59 [0.40-0.89], p =0.01). However, no differences were found in terms of ST (OR [95% CI] =0.90 [0.47-1.74], p =0.77), cardiac death (OR [95% CI] =1.07 [0.60-1.92], p =0.80) and recurrent MI (OR [95% CI] =0.84 [0.49-1.47], p =0.55).
Discussion
The major findings of our meta-analysis are as follows: 1) in STEMI patients undergoing PCI, DES reduce the incidence of TVR and recurrent MI up to 1 year, 2) DES use is not associated with an increased risk of acute to late ST or cardiac death, and 3) SES use is associated with a higher TVR reduction by indirect comparison with PES.
Over the last few years, growing concerns have been raised about DES safety and efficacy, especially in STEMI patients.28 In the present study, a 27% reduction in the odds of recurrent MI was achieved with DES use as compared with BMS. Recently, several retrospective studies have been published on this issue, showing a benefit of DES over BMS in terms of repeat revascularisation and mortality.29,30 On the contrary, the GRACE registry reported an increased 2-years morality rates in patients previously treated with DES.31 In addition, one trial reported a tendency towards increased cardiac mortality with DES17. Previous meta-analyses on this topic, with smaller samples size, failed to demonstrate a benefit of DES over BMS in terms of recurrent MI.32,33
We found significantly lower rates of TVR in DES group, with a 57% odds reduction. In addition, meta-regression showed that with an increased risk of clinical restenosis, considered as the incidence of TVR in BMS patients, there was a parallel increase of clinical benefit with the use of DES, suggesting that the higher the risk of TVR, the bigger the benefit from DES use (Figure 3). In turn, the remarkable reduction in TVR might contribute to explain the significant reduction in recurrent MI observed in patients undergoing DES implantation. In fact, it’s well known that restenosis is not a benign clinical entity, since it has been showed to be an independent risk factor for mortality, with an absolute increase in mortality of about 3% in patients developing restenosis as opposed to patients without restenosis.34 Furthermore, MI and unstable angina have been found to be the clinical presentation of restenosis in more than one third of patients,35 with MI presentation associated with a greater risk of death as compared with MI presentation in absence of an underlying restenosis.36 Finally, the treatment of in-stent restenosis may be complicated by peri-procedural MI.37 In the present study, cumulative incidence of acute to late ST did not differ within DES and BMS groups. This is a main safety finding, since in acute MI clinical scenario, a large thrombotic burden, suboptimal stent expansion and malapposition, as a consequence of huge thrombus resolution, could play a pivotal role in the pathophysiology of ST.
The adjusted indirect comparison further expands our findings. This statistical technique does not replace RCTs of direct comparison but it could be useful to strengthen the power of comparisons when few direct studies are available. At this regard, to date there is only one head-to-head and small-sample sized trial of SES and PES in primary PCI, that did not meet any significant difference between SES and PES at 12-month.38 Similarly, this meta-analysis did not find any difference between two DES type in terms of ST, cardiac death or recurrent MI, showing, however, a greater TVR reduction obtained with SES as compared to PES.
This meta-analysis presents several limitations. This is a meta-analysis at the study level, and we could not properly assess the role of confounding factors. In addition, this meta-analysis does not provide follow-up data longer than 1-year, that are crucial to evaluate very late stent thrombosis incidence. However, 2-year follow-up of the PASSION,39 4-year follow-up of the TYPHOON40 and 5-year follow-up of the STRATEGY41 showed a persistent benefit from DES implantation in terms of repeat revascularisation and no difference considering very late stent thrombosis incidence.
In conclusion, this meta-analysis demonstrates that in patients undergoing primary angioplasty, treatment with DES is associated with decreased myocardial infarction and target-vessel revascularisation rates without increasing stent thrombosis and death, up to 1-year. Indirect comparison revealed SES as associated with a higher reduction in target-vessel revascularisation as compared with PES. Longer follow-up results of included trials, and appropriately powered direct comparison between SES and PES are needed to confirm a sustained benefit over time from DES implantation, and the superiority of SES over PES in terms of TVR reduction.

