Abstract
Drug-eluting stents (DES) dramatically lower rates of restenosis in patients undergoing percutaneous coronary interventions. However, their use has extended beyond the initial approved indications into off-label scenarios such as ST-elevation myocardial infarction (STEMI). Multiple retrospective case series and 10 prospective randomised controlled trials have been published on the use of DES in STEMI patients, and these generally show reduced rates of target vessel revascularisation with a neutral effect on mortality. Three meta-analyses show similar findings with the composite endpoint of death, recurrent myocardial infarction, or re-intervention significantly lower in the DES group, although this was largely driven by a decrease in target vessel revascularisation. These studies also demonstrate that DES use in the setting of STEMI is not associated with increased risk of stent thrombosis compared to bare metal stents. In summary, current data support the efficacy of DES in reducing restenosis and re-intervention in patients who present with STEMI. From a safety standpoint, the use of DES in STEMI does not increase the risk of stent thrombosis, and there is no difference in overall mortality. The use of DES in STEMI patients should be individualised on a case-by-case basis given the importance of compliance with dual antiplatelet therapy.
Introduction
The development of drug-eluting stents (DES) represents an important milestone in the history of percutaneous coronary intervention with dramatic reductions in the rates of restenosis and target lesion revascularisation (TLR). However, the use of DES has recently come under intense scrutiny beginning in 2006 with reports suggesting that DES in general, were associated with a higher risk of late stent thrombosis (LAST) and perhaps increased mortality1,2.
In addition, the pivotal trials for the US-approved DES involved a strictly defined group of patients and indications, and did not include ST-elevation myocardial infarction (STEMI) patients. However, after regulatory approval, reports began to emerge on DES use in off-label indications, including high risk subsets such as STEMI. The question of whether DES use in the setting of STEMI would predispose to early or late stent thrombosis (ST) and increased risk of subsequent myocardial infarctions then arose3,4. The following review examines the available body of literature for the use of DES in the setting of STEMI.
Stent thrombosis in STEMI patients receiving DES
In general, acute coronary syndromes are associated with a higher risk of stent thrombosis (ST) in both BMS5 and DES6. In STEMI patients, possible reasons for this include the presence of thrombus, higher rates of late stent malapposition, enhanced resistance to antiplatelet agents and a pro-inflammatory state (Figure 1).
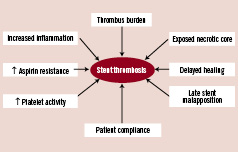
Figure 1. Factors contributing to increased risk of stent thrombosis in STEMI patients treated with DES.
Some authors have suggested that delayed healing, lack of endothelialisation, presence of the exposed necrotic core to flowing blood, and enhanced platelet reactivity lead to the increased risk of ST in acute coronary syndromes7. The presence of thrombus has also been shown to be an independent predictor of ST8, and in addition, Sianos and colleagues showed that a large thrombus burden was associated with major adverse cardiac events (MACE) and ST9. Primary PCI for STEMI has been implicated as a predictor of late stent malapposition which can occur in up to 31.8% of primary PCI using DES, compared to 11.5% in BMS10. STEMI, in addition, is associated with aspirin resistance11, and increased inflammation12.
Registry studies
The use of sirolimus eluting stents (SES) in STEMI was first reported by Saia et al in 200313. A follow-up study from the same group examined a larger cohort of patients who had either BMS or DES. In this non-randomised study, 186 patients received SES compared to 183 who received a variety of BMS14. There were no differences in 30-day outcomes. The Thoraxcenter investigators then compared the cohort of 186 patients who received SES with a subsequent group of 136 patients who received paclitaxel-eluting stents (PES) in the setting of STEMI15. At 30 days and one year, there were no significant differences in mortality or reinfarction between the two groups.
While the short term results from registry studies favour the use DES in STEMI, at least in terms of revascularisation rates, this advantage has not been shown in long term studies. Daemen et al reported 3-year data from both the RESEARCH and T-SEARCH registries at the Thoraxcenter. Of the 505 patients reported, 183 had BMS, 186 had SES and 136 had PES16. While there was no significant difference in the rates of major cardiac events, the incidence of ST trended higher in both DES groups (BMS 1.6%, SES 2.7%, PES 2.9%; p=ns for all). Importantly, target vessel revascularisation (TVR) rates were similar in all groups (BMS 12.0%, SES 11.5%, PES 12.4%).
At the 2007 European Society of Cardiology meeting, Steg et al presented results from the Global Registry of Acute Coronary Events (GRACE) registry in death rates following the use of DES (n=569) for STEMI was compared to BMS (n=1729) use. The investigators found that while there were no significant differences in deaths up to 180 days, the death rate between 180 days and 730 days was significantly higher in DES-treated patients, even after multiple regression analyses17. It should be noted that the findings of the GRACE registry have not been formally published. Finally, Hannan et al published data from the New York State’s PCI registry which favoured DES, demonstrating that the use of DES (n=1154) in STEMI was associated with lower risk-adjusted mortality compared to BMS (n=772) (5.0% vs. 8.6%, p=0.007)18.
In summary, registries and retrospective studies show inconclusive data regarding the safety of DES use in STEMI patients. Most studies, with the exception of the GRACE registry, show similar outcomes in reinfarction and death. To provide more definitive data, several randomised trials were performed and are reviewed below.
Randomised controlled trials
There are currently 10 published randomised trials comparing DES to BMS in STEMI (Table 1 and Figure 2).
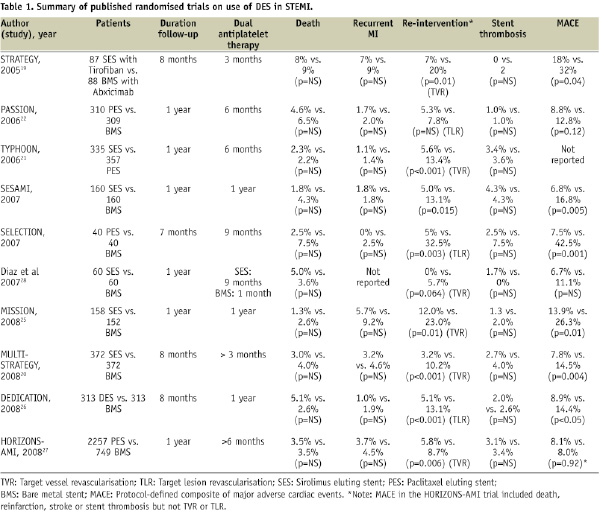
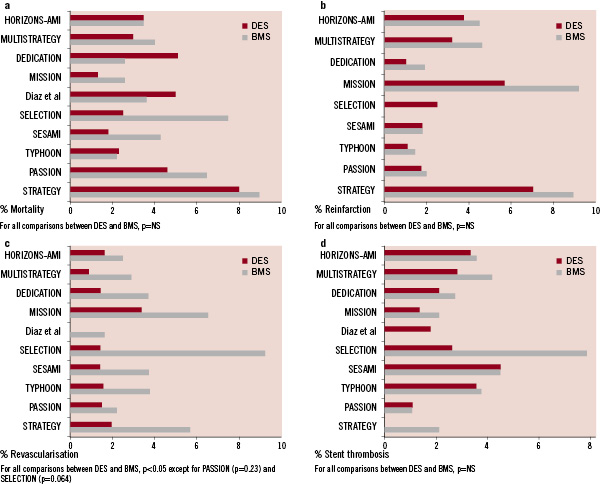
Figure 2. a. Mortality for DES and BMS in randomised controlled trials for STEMI. All outcomes at one year; except for SELECTION at seven months, STRATEGY and MULTISTRATEGY at eight months. b. Reinfarction for DES and BMS in randomised controlled trials for STEMI. All outcomes at one year; except for SELECTION at seven months, STRATEGY and MULTISTRATEGY at eight months. Diaz et al did not report reinfarction rates. c. Repeat revascularisation for DES and BMS in randomised controlled trials for STEMI. All outcomes at one year; except for SELECTION at seven months, STRATEGY and MULTISTRATEGY at eight months. d. Stent thrombosis for DES and BMS in randomised controlled trials for STEMI. All outcomes at one year; except for SELECTION at seven months, STRATEGY and MULTISTRATEGY at eight months.
The STRATEGY trial randomised 175 patients to either the use of tirofiban and SES or the use of abciximab and BMS19. The study was designed to assess the relative efficacy of SES versus BMS in STEMI and to evaluate whether the higher cost of SES could be reduced by a less expensive glycoprotein IIb/IIIa inhibitor. While the 8-month primary combined endpoint of death, non-fatal myocardial infarction, stroke and binary stenosis was lower in the SES/tirofiban group, this was mainly due to the lower binary restenosis rates in the SES group. The investigators subsequently performed the stratified MULTISTRATEGY study in which patients were first randomised to either tirofiban or abxicimab, and then to either SES or BMS20. At eight months, the incidence of major adverse cardiac events was significantly lower in the SES group (7.8%) than in the BMS group (14.5%), again mainly due to reduction in repeat revascularisation rates. The incidence of stent thrombosis was similar in the two stent groups. Interestingly, patients and investigators were not blinded in these two studies, although primary outcomes events were blindly adjudicated.
The TYPHOON study was a multicentre trial including 712 patients randomised to either SES or BMS21. The primary endpoint, a composite of target-vessel-related death, recurrent myocardial infarction, or TVR at one year was significantly lower in the SES group than in the BMS group (7.3% vs. 14.3%, P = 0.004). Like the STRATEGY study, this reduction was driven by a decrease in the rate of TVR, which could have been influenced by the routine angiographic follow-up. No significant differences between the groups were found in the rates of death, reinfarction or ST.
The PASSION trial involved 619 patients in two centres and compared PES to BMS in STEMI patients22. The primary endpoint, a composite of death from cardiac causes, recurrent myocardial infarction, or TLR at one year, trended towards superiority in the PES group (adjusted relative risk 0.63, p=0.09). There was no significant difference in the rates of death from cardiac causes or recurrent myocardial infarction, TLR or ST. Of note, in the PASSION trial, no routine angiographic follow-up was performed22. The recently presented 2-year results demonstrated a continuing trend towards lower MACE rates in the PES group (driven by lower TVR rates) (PES 11.1% vs. BMS 15.4%, p=0.12), while there were no differences in rates of death, recurrent myocardial infarction or angiographically proven ST23.
The randomised, single centre SESAMI trial (n=320) compared the use of SES vs. BMS in STEMI24. At one year, the incidence of the primary endpoint, binary restenosis, was significantly lower in the SES group than in the BMS group (9.3% vs. 21.3%, p=0.032), subsequently resulting in lower rates of TLR and TVR. There were also no significant differences in the mortality, reinfarction rates or ST.
The MISSION study was also a single centre randomised trial comparing SES to BMS in 310 patients with STEMI25. The primary end point was in-segment late luminal loss at nine months with late stent malapposition on intravascular ultrasound as a secondary endpoint. Clinical events were reported at 12 months. At nine months, in-segment late luminal loss was lower in the SES group. There was a higher rate of late stent mal-apposition in the SES group compared to BMS group (37.5% vs. 12.5%, p<0.001), which did not translate to increased MACE. Revascularisation rates, even when clinically driven, were lower in the SES group compared to the BMS group at 12 months (2.5% vs. 7.9%, p=0.03). Overall, there were no differences in the rates of death, myocardial infarction or ST.
The DEDICATION trial (n=626) randomised subjects who presented with STEMI to either DES or BMS in a 2x2 factorial design trial; and to the use of distal protection device or not26. The study was not blinded to patients or investigators. The primary endpoint, mean late lumen loss, was significantly lower in patients treated with a DES. The rate of the composite end point of cardiac death, reinfarction, and TLR was 8.9% in the DES group versus 14.4% in the BMS group (p=0.03). Of note, death occurred in 5.1% vs. 2.6% (p=NS) respectively.
The HORIZONS-AMI trial was one of the largest contemporary interventional STEMI trials. It had a 2x2 factorial design randomising patients to bivalirudin versus heparin and a glycoprotein IIb/IIIa antagonist, and a second randomisation to either PES or BMS27. In this study of 3,602 patients enrolled from 123 centres in 11 countries, 2,257 patients were randomised to PES while 749 were randomised to BMS in a 3:1 ratio. The study showed no difference at 12 months in the overall MACE rate (8.1% vs. 8.0%) or its components of death, reinfarction, ST and stroke. Ischaemia driven TLR was significantly lower in the PES group (4.5% vs. 7.5%, p=0.002).
Smaller studies by Diaz et al28 and Chechi et al (SELECTION trial)29 similarly showed no differences in death, reinfarction or ST between DES and BMS.
In general, these randomised trials show substantial benefits in reducing repeat revascularisation, without any significant difference in death, stent thrombosis or reinfarction. With the exception of the STRATEGY trial which reported no difference in death, stent thrombosis and reinfarction at two years, even after discontinuation of clopidogrel, most other trials have yet to report long term follow-up.
Meta-analyses
There have been three meta-analyses of randomised trials of DES in STEMI30-32. In Pasceri’s meta-analysis, seven randomised trials were examined including a total of 2,357 patients (1,177 with DES and 1,180 with BMS). No evidence of heterogeneity between the trials was found. MACE occurred in 9.3% for the DES group compared to 17.6% in the BMS group (relative risk (RR) 0.53, 95% CI 0.43 to 0.66). Mortality data at 1-year, available for five trials, were similar between the two groups (DES 2.8% vs. BMS 3.1%). Reinfarction was also similar between the two groups. Consistent with other patient populations, TLR occurred in 4.8% of DES and in 12.0% of BMS patients (RR 0.40, 95% CI 0.30-0.54). ST rates were similar in both groups with 2.3% in DES and 2.6% in BMS patients.
Kastrati’s meta-analysis comprised eight randomised trials, including six of those described in Pasceri’s meta-analysis31. In contradistinction to the previously described meta-analysis, Kastrati and colleagues were able to obtain patient level data from the investigators in seven of the eight studies (all except the MISSION trial). There were 2,786 patients in the eight trials. There was no significant heterogeneity between the trials. The composite endpoint of death, recurrent myocardial infarction, or re-intervention was 9.5% in the DES group and 17.8% in the BMS group (hazard ratio 0.53, p<0.001). As expected, the main driver for differences in the composite endpoint was the need for re-intervention with rates of 5.0% in the DES group and 13.3% in the BMS group (hazard ratio 0.38, P<0.001). ST rates at one year were similar in both groups (DES 1.6%, BMS 2.2%). Recurrent myocardial infarction rate was 2.5% in the DES group compared to 3.3% in the BMS group (hazard ratio 0.72, p=0.11). Overall, the use of DES was associated with a hazard ratio of 0.76 for death (DES 4.0%, BMS 5.0%, p=0.14). The authors repeated their analysis excluding the MISSION study for which they did not have access to patient level data and found no meaningful difference in the results.
The most recent meta-analysis by De Luca et al included 11 trials, but not the HORIZONS-AMI trial30. A total of 3,605 patients were included in this analysis and found no significant difference in mortality (4.1% vs. 4.4%, p=0.59), reinfarction (3.1% vs. 3.4%, p=0.38) or stent thrombosis (1.6% vs. 2.2%, p=0.22), whereas DES were associated with a significant reduction in TVR (5.0% vs. 12.6%, p<0.0001).
Discontinuation of dual antiplatelet therapy
While there are multiple risk factors associated with ST, premature discontinuation of dual antiplatelet therapy (more specifically, clopidogrel therapy) is probably the single most important risk factor33-36. Unfortunately, the existing literature does not consistently define what constitutes an adequate duration of dual-antiplatelet therapy, and therefore, it is difficult to define premature discontinuation of therapy. For example, in the report by Iakovou et al, aspirin was prescribed indefinitely while clopidogrel was prescribed for three months in SES and six months in PES34. In general, most of the early reports describe the use of clopidogrel for at least three to six months. Importantly, Airoldi et al found that the association between discontinuation of dual antiplatelet therapy and ST appears to be the strongest in the first six months33. In this study, after six months, discontinuation of clopidogrel was not a significant predictor of ST.
Although dual antiplatelet therapy is currently recommended after implantation of DES for a minimum of 12 months37, there is no certainty regarding an individual patient’s medical compliance. In the PREMIER registry, 13.6% of 500 patients with myocardial infarctions treated with DES stopped thienopyridines within 30 days36. These patients had a dramatically higher risk of mortality at one year compared to compliant patients (7.5% vs. 0.7%, p<0.0001). Even though the adjusted hazard ratio for ST in this study was 9.0, other studies have reported up to a 90 fold increase in the risk of ST34. Furthermore, even in randomised controlled trials such as the MULTISTRATEGY trial, compliance was only ~50% at one month even though the protocol required a minimum of three months of therapy20. Although beyond the scope of this paper, other factors such as genetic polymorphisms, aspirin and clopidogrel resistance are future targets for investigation in predicting ST.
Treatment of STEMI due to stent thrombosis
There are limited data on the use of DES in patients who present with STEMI resulting from ST. Regardless of whether BMS or DES is used; data is scarce as to the optimal treatment strategy. Available studies report a high rate of mortality with ST associated with DES34,35,38 with some reporting up to 45% 6-month mortality34. Ong et al, from the Thoraxcenter, reported their experience treating seven patients with eight late ST events (defined as occurring >30 days after the index procedure). In their series, two had thrombectomy, all had balloon angioplasty and seven of the eight events were treated with another DES. There were two deaths in this report39. The OPTIMIST registry, which is a prospective multicentre European registry for ST, recently reported their initial findings at the 2007 European Society of Cardiology Congress meeting and showed a 6-month mortality rate of 17%, and a 29% rate of major adverse coronary or cerebral events (death or myocardial infarction or stroke or re-intervention)40. These results suggest that ST, even when treated with emergent PCI, is still associated with significant mortality and morbidity. However, detailed findings such as the treatment strategy and types of stents used will have to await formal investigation.
Unresolved issues
The randomised clinical trials described above have important inclusion and exclusion criteria that may potentially limit widespread applicability. For example, patients with cardiogenic shock, rescue PCI pos-thrombolysis, and late presentation are often excluded. The currently ongoing multicentre randomised EXAMINATION (A Clinical Evaluation of Xience-V stent in Acute Myocardial Infarction) trial, which compares the use of the Xience-V stent to the Vision stent, will address some of these issues by including all STEMI patients up to 24 hours of presentation (including patients with cardiogenic shock).
Another important issue is the use of DES patients who present with stent thrombosis. Current data remain insufficient to determine the appropriate strategy when choosing stent type (e.g. DES vs. BMS) in these patients. While randomised trials are unlikely to have sufficient patients, prospective registry studies may provide useful insights into the mechanism and optimal management of patients who present with stent thrombosis, including the choice of stents.
Conclusion
Currently available data support the efficacy of DES in preventing restenosis and re-intervention in patients who present with STEMI. From a safety standpoint, the use of DES in STEMI has recently shown to be associated with no increased risk of ST, and no difference in the risk of overall mortality. This is balanced against the reduced need for revascularisation compared to BMS. Why then, despite the data summarised in this review, do many physicians feel that DES should be used with caution in STEMI? Firstly, the use of DES may put the patient at increased risk of death from ST if prolonged access to clopidogrel is not available or if medical compliance is an issue. Second, despite the clear superiority of DES in randomised controlled trials, registry studies, such as those reported from the RESEARCH and T-SEARCH registries, suggest that early benefits in lowering revascularisation rates are diminished during long term follow-up such that MACE rates are similar among patients with DES and BMS16,41.
A major risk factor for ST appears to be premature discontinuation of dual antiplatelet therapy. Although there are limited data, the ACC/AHA/SCAI have recommended at least one year of dual antiplatelet therapy37. Therefore, as long as a patient has access to, and is compliant with dual antiplatelet therapy, the use of DES would provide the benefits of reduced restenosis without the increased risk of reinfarction and death.

