Abstract
Aims: The use of drug-eluting stents (DES) in patients with non-ST-segment elevation acute coronary syndrome (NSTE-ACS) is controversial and not yet endorsed in clinical guidelines.
Methods and results: This was an a priori planned post hoc analysis involving 754 NSTE-ACS patients from the randomised BASKET-PROVE trial (sirolimus-eluting stent vs. everolimus-eluting stent vs. bare metal stent in large-vessel stenting). The primary endpoint was the combined two-year rate of cardiovascular death or non-fatal myocardial infarction (MI). Secondary endpoints were each component of the primary endpoint, and clinically indicated target vessel revascularisation (TVR) and stent thrombosis. Compared to patients with BMS, those treated with SES and EES had a strong trend towards lower two-year rates of the primary endpoint (HR: 0.31 [CI: 0.11-0.90], p=0.03, and HR: 0.74 [CI: 0.44-1.24], p=0.25), and of TVR (HR: 0.58 [CI: 0.29-1.15], p=0.12) and (HR: 0.52 [CI: 0.34-0.78], p=0.002). When the SES and EES groups were combined and compared to BMS, significant reductions in both cardiovascular death/MI and TVR were found.
Conclusions: Compared with BMS, use of DES in NSTE-ACS patients undergoing stent implantation in large vessels was associated with a reduction in both TVR and the combined endpoint consisting of cardiovascular death/MI. Thus, DES use improves both efficacy and safety. These findings support the use of DES in NSTE-ACS patients.
Introduction
Percutaneous coronary intervention (PCI) is the optimal treatment for patients admitted with non-ST-segment elevation acute coronary syndromes (NSTE-ACS)1-3. However, in recent years it has been debated whether drug-eluting stents (DES) or bare metal stents (BMS) provide better outcomes for NSTE-ACS patients often presenting with a ruptured plaque. The main purpose of PCI is to restore flow rapidly in the occluded/stenotic artery to reduce myocardial damage and, on a long-term basis, to ensure that the vessel remains open. However, during follow-up, in-stent restenosis is a substantial problem in patients treated with BMS, while the use of DES may be associated with an increased risk of the potentially lethal late stent thrombosis (ST)4,5. DES were originally designed to reduce the risk of in-stent restenosis, without compromising safety. Recommendations regarding DES were largely based on experiences from patients with stable coronary artery disease6-8. However, lesion pathology in patients with NSTE-ACS is different compared to patients with stable plaques, and the risk of stent thrombosis (particularly very late stent thrombosis), reinfarction and death may be increased in NSTE-ACS patients treated with DES instead of BMS9,10. This may in part be due to the following mechanisms: the ruptured plaque enables the drug to diffuse directly into the vessel wall increasing the risk of inflammation, increased hypersensitivity in the arterial wall11, lack of appropriate re-endothelialisation and delayed intimal healing12-14. Furthermore, the presence of thrombus material may complicate stent delivery leading to underexpansion/malapposition15,16. Finally, DES seem to induce a more pronounced positive remodelling of the vessel, increasing the risk of stent malapposition17,18.
Despite this potential lethal complication, current data indicate that long-term safety (mortality and new myocardial infarction) is similar in DES and BMS-treated NSTE-ACS patients, with a trend towards improved outcome in DES patients. Furthermore, it now seems well established that DES reduces the need for target lesion/vessel revascularisation (TLR/TVR) compared to BMS19-22. Based on these experiences, the use of DES in ACS patients has increased significantly (>60% of all procedures)23. This raises an interesting question regarding the stent choice in NSTE-ACS patients: if DES use primarily reduces the need for TVR without significantly improving safety on a long-term basis, is it then worth the additional costs related to DES implantation? In a recent Cochrane review aiming to answer that question the answer was “no”24.
The use of DES in patients with NSTE-ACS is controversial and currently not endorsed in the guidelines and as such classified “off-label”1. The following statements are presented in the latest (2011) ESC guidelines evaluating this issue: “The safety and efficacy of DESs have not been prospectively tested in this specific population (NSTE-ACS)” and “the choice between the use of a BMS or a DES should be based on an individual assessment of benefit and risk”1. In this study we focused on both efficacy and long-term safety (in terms of cardiovascular death or non-fatal MI) in a contemporary NSTE-ACS population treated with either DES or BMS.
Methods
STUDY DESIGN
This was an a priori planned substudy from the BASKET-PROVE trial. The study design of BASKET-PROVE has previously been described in detail25. In brief, BASKET-PROVE was a multicentre randomised investigator-driven clinical trial. Financial support was given by the Basel Cardiovascular Research Foundation, the Swiss National Foundation for Research and the Swiss Heart Foundation. The protocol was approved by the ethics committee at each centre and each patient gave written informed consent. The authors have full access to the data and take responsibility for its integrity. All authors have read and approved the manuscript as written.
STUDY POPULATION
Patients were included at the participating centres in Switzerland, Denmark, Austria and Italy between March 5, 2007, and May 15, 2008. Patients were all-comers with chronic or acute coronary disease treated with PCI and in need of stenting in vessels ≥3.0 mm in diameter. Exclusion criteria were cardiogenic shock, in-stent stenosis, stent thrombosis, unprotected left main coronary disease, planned surgery within 12 months, a need for oral anticoagulation, an increased risk of bleeding and suspected non-compliance with long-term antiplatelet therapy.
All 754 patients presenting with NSTE-ACS were considered for this analysis.
STUDY PROCEDURES
Patients were randomised in a 1:1:1 ratio to a first-generation sirolimus-eluting stent (SES) (Cypher Select®; Cordis, Johnson & Johnson, Warren, NJ, USA), a bare metal (cobalt-chromium) stent (BMS) (Vision®; Abbott Vascular, Santa Clara, CA, USA), or a second-generation everolimus-eluting stent (EES) (XIENCE V®; Abbott Vascular). Angioplasty, stenting and secondary medical prevention were performed and given according to current techniques and guidelines at the operator’s discretion. However, all patients were prescribed aspirin at a daily dose of 75-100 mg and clopidogrel at a daily dose of 75 mg for one year after a loading dose of 600 mg regardless of stent type. Patients with multiple lesions in need of more than one stent were treated with the same type of stent for all lesions.
ENDPOINTS AND DEFINITIONS
The primary endpoint for this subgroup analysis was the combination of two-year rates of cardiovascular (CV) death or non-fatal MI (the same primary endpoint used in the primary BASKET-PROVE trial). Secondary endpoints were each of the components of the primary endpoint separately and clinically indicated target vessel revascularisation and ST. CV death was defined as death with a definite cardiac cause or death without any clear extra-cardiac cause. Non-fatal MI was defined as a clinical event with typical ECG and enzymatic changes4. TVR and ST were defined according to the Academic Research Consortium definitions5. Definite and probable ST are used in these analyses. Follow-up angiography was not mandated and only performed if clinically indicated. All endpoints were adjudicated by an independent events committee blinded to the stent type used.
STATISTICAL ANALYSES
Baseline characteristics were compared using the χ2 test and continuous Gaussian distributed variables with the Student’s unpaired t-test or Kruskal-Wallis test. The analyses of outcome were performed according to the “intention-to-treat” principle. Outcome is presented as hazard ratios after one and two years of follow-up. Outcome is presented both with unadjusted Cox regression analyses and adjusted multivariable Cox backward-conditional analyses (entry: p<0.25 and removal: p<0.10). All variables in Table 1 were initially entered in the adjusted models. Results from the final step are presented in the paper. The assumption of linearity and proportional hazards in this model was assured. First order interactions between the main stent variable and other baseline variables for endpoints were assessed in Cox models. In order to maintain a robust model, only one variable per each five events was allowed in the final multivariable Cox analysis. Time-dependent unadjusted Kaplan-Meier plots were compared using the log-rank test. As a post hoc analysis the SES and the EES groups were merged into one DES group and compared to the BMS group.
In the statistical test, p-values ≤0.05 were considered of statistical significance. IBM SPSS for Windows version 20.0 (IBM Corp., Armonk, NY, USA) was used.
Results
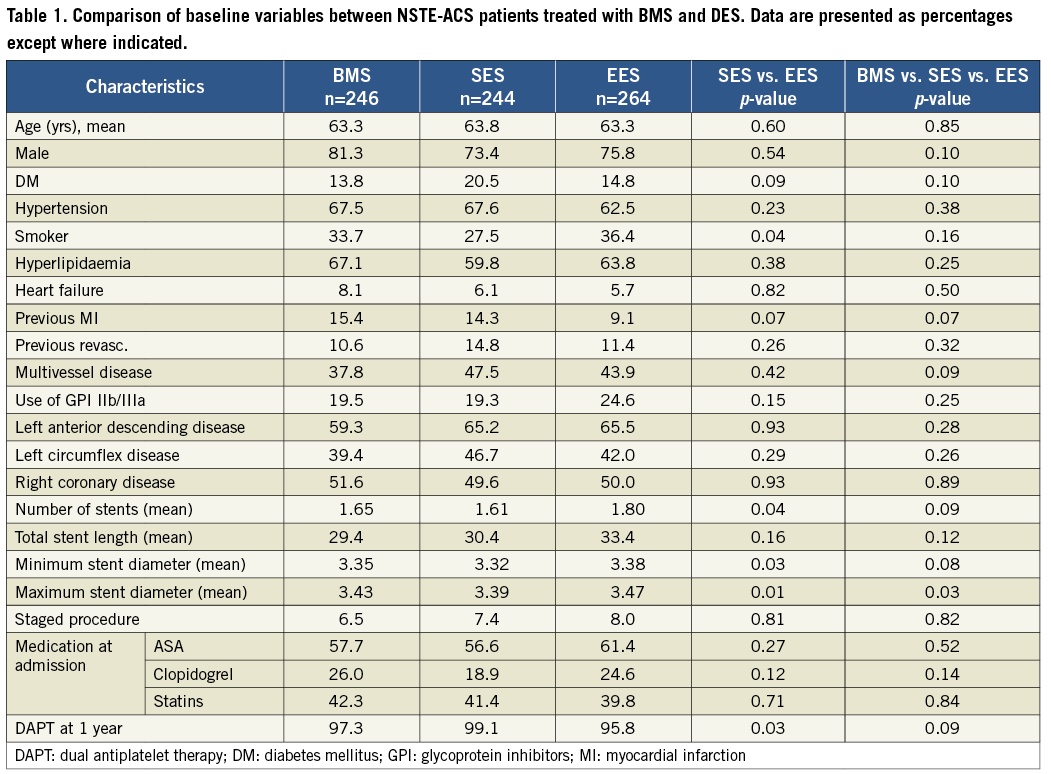
PATIENT POPULATION AND BASELINE CHARACTERISTICS (TABLE 1)
A total of 754 patients from the BASKET-PROVE trial were included with the indication NSTE-ACS (754/2,314=33%). All were treated with PCI (246 had a BMS, 244 a SES and 264 an EES). No combination was used. No difference was found between the two DES groups apart from a slightly larger stent burden in the EES group and larger proportion of use of dual antiplatelet therapy (DAPT) (ASA+clopidogrel) at one year in the SES group.
When all three stent groups were compared, no significant baseline differences were found apart from a difference in maximum stent diameter. This indicates that randomisation was also successful in the NSTE-ACS subgroup.
ADJUSTED OUTCOME ANALYSES (TABLE 2 AND FIGURE 1)
SES vs. EES: no significant differences were found between the SES and the EES groups, although a trend towards a reduced risk of TVR in the EES group was found after two years (HR: 0.56 [CI: 0.23-1.35], p=0.20).
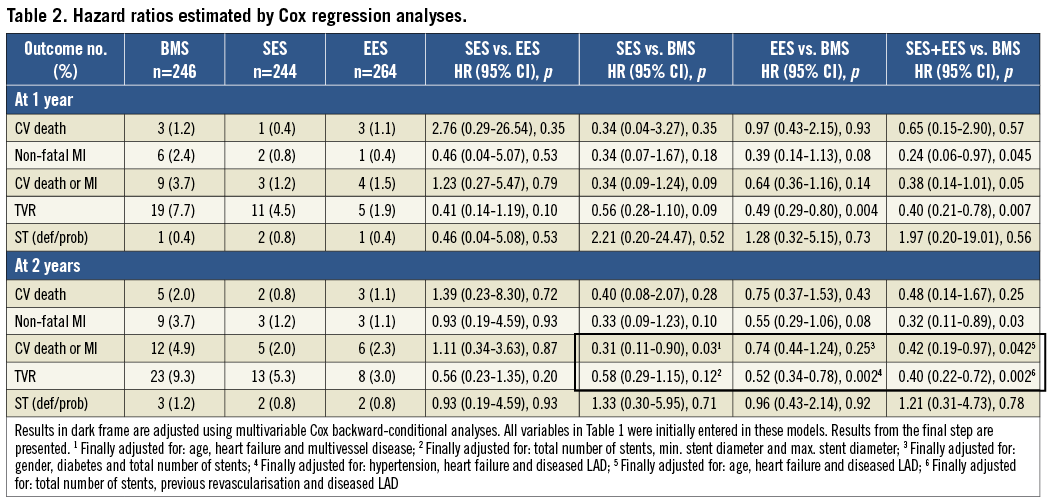
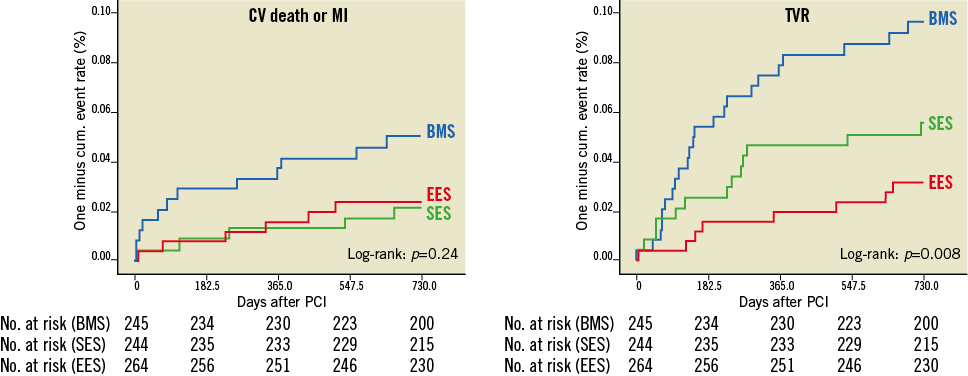
Figure 1. Kaplan-Meier plots for the combined endpoint of CV death or non-fatal MI and TVR. Comparison between stent groups by log-rank test (n=754).
SES vs. BMS: patients treated with SES had a significantly lower risk of CV death/MI (HR: 0.31 [CI: 0.11-0.90], p=0.03), and a trend towards reduced risk of TVR in the SES group was also found (HR: 0.58 [CI: 0.29-1.15], p=0.12).
EES vs. BMS: for EES patients, only a trend towards reduced risk of CV death/MI was found (HR: 0.74 [CI: 0.44-1.24], p=0.25). However, EES patients had a significantly reduced risk of TVR (HR: 0.52 [CI: 0.34-0.78], p=0.002).
SES+EES vs. BMS: when the SES and the EES groups were merged into one DES group, a significant reduction in both CV death/MI and TVR was found for DES (HR: 0.42 [CI: 0.19-0.97], p=0.042 and HR: 0.40 [CI: 0.22-0.72], p=0.002). Although a trend towards a reduced risk of CV death was found, the reduction in the primary endpoint (CV death/MI) in the DES group was primarily driven by a significant reduction in non-fatal MI (unadjusted HR: 0.32 [CI: 0.11-0.89], p=0.03).
In general, two thirds of all endpoints occurred within the first year. No differences in terms of ST were found in any of the analyses. However, only a very small number of ST occurred in the BASKET-PROVE trial.
Discussion
Compared with BMS, the use of DES in NSTE-ACS patients undergoing stent implantation in large vessels was associated with a reduction in both TVR and the combined endpoint consisting of cardiovascular death/MI. Thus, DES use improves both efficacy and safety. These findings support the use of DES in NSTE-ACS patients.
Currently, it seems well established that DES are superior to BMS in terms of reducing the risk of TVR, without compromising safety19-22. However, randomised controlled trials (RCTs) including the subgroup of patients with NSTE-ACS have failed to demonstrate the superiority of DES in terms of safety outcome23,24. In some observational studies, DES use has demonstrated favourable outcome in terms of all-cause mortality and non-fatal MI20,23, while others only demonstrated the superiority of DES in terms of reducing TVR whereas safety outcomes were unaffected by stent choice19,21. Still, due to a lack of randomised data comparing DES and BMS use in NSTE-ACS populations, DES use has not been endorsed in recent European guidelines1. Furthermore, even though DES is superior in terms of reducing TVR, but maybe only equal to BMS in terms of safety, cost/benefit issues could be and have been raised regarding DES use in this high-risk population. A recent Cochrane review concluded that “increased cost of drug-eluting stents and lack of evidence of their cost-effectiveness means that various health funding agencies are having to limit or regulate their use in relation to price premium”24. In this context, we believe that our results add very important knowledge, since improved survival and freedom from MI obviously alter the cost/benefit balance in favour of DES. To our knowledge, this is the first time that long-term data from an RCT have demonstrated this association. Possible explanations for the improved outcome in safety may be that the DES used in this trial were superior to other DES. The XIENCE stent (EES) (Abbott Vascular) is a second-generation stent with thinner struts, biocompatible polymers and less aggressive drug doses. Furthermore, substantial data suggest that the first-generation sirolimus-eluting stent (SES) (Cypher; Cordis) used in this study is superior to the paclitaxel-eluting stent used in prior comparisons of ACS patients19,26-28. This may also explain why no significant differences between the two drug-eluting stents were found in the present NSTE-ACS subgroup analysis or in the main BASKET-PROVE trial. Still, we did find a trend towards a reduced risk of TVR in the EES group compared to the SES group, even though a greater number of stents as well as larger stents were used in the EES group, thus increasing the stent burden. However, this association seems to be in concordance with other data suggesting that EES are superior to SES in terms of efficacy (with no differences in safety outcome)29. Perhaps a more important aspect is that the majority of procedures were performed in high-volume PCI centres with a focus on avoiding underexpansion and with extensive use of post-dilation. Efforts to reduce malapposition are particularly important when implanting DES, since underexpanded DES are less likely to be re-endothelialised compared to BMS, and therefore much more thrombogenic12. Finally, our study only focused on patients in need of stenting in vessels ≥3.0 mm in diameter. DES may be less effective in large vessels in terms of both efficacy and safety outcome. Actually, this concern was raised based on findings from the first Basket Trial30, and the recent BASKET-PROVE trial was initiated to investigate this matter further31. Convincingly, this concern has also now been eliminated in NSTE-ACS patients. Still, our findings should not be extrapolated uncritically to smaller vessel stenting.
Study limitations
This study was – although a priori planned – a post hoc analysis of the subgroup of NSTE-ACS patients from the BASKET-PROVE trial. The study design (i.e., randomisation and power calculations) was not focused on the NSTE-ACS subgroup. However, only a few differences in baseline variables were found, indicating that randomisation was successful. In this subgroup analysis (as well as in the primary BASKET-PROVE trial) event rates were, fortunately, rather low, which may have induced a risk especially of type 2 errors. Possible explanations for the low event rates may be: recommendation (and high compliance) of DAPT up until one year, high-volume centres, and also the fact that only patients in need of stenting in vessels ≥3.0 mm in diameter were included. Finally, as in all RCTs, our results may not directly be extrapolated to all real-life patients presenting with NSTE-ACS: in particular, patients with an expected low compliance for antiplatelet therapy and those with a high bleeding risk (who were excluded from this study) have to be evaluated carefully before treatment with a DES.
Conclusion
Compared with BMS, the use of DES in NSTE-ACS patients undergoing stent implantation in large vessels was associated with a reduction in both TVR and the combined endpoint consisting of cardiovascular death/MI. Thus, DES use improves both efficacy and safety. To our knowledge, this is the first time that long-term data from an RCT have convincingly demonstrated this association, and these findings strongly support the use of DES in NSTE-ACS patients.
| Impact on daily practice The use of drug-eluting stents (DES) in patients with non-ST-segment elevation acute coronary syndrome (NSTE-ACS) is controversial and not yet endorsed in clinical guidelines. The aim of this study was to elucidate further whether DES or BMS is optimal in this high-risk ischaemic population. Compared with BMS, use of DES in NSTE-ACS patients undergoing stent implantation in large vessels was associated with a reduction in both TVR and the combined endpoint consisting of cardiovascular death/MI. Thus, DES use improves both efficacy and safety. These findings support the use of DES in NSTE-ACS patients. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

