Abstract
Aims: The aim of this substudy of the SORT OUT IV trial was to compare clinical outcomes among patients with acute coronary syndromes (ACS) and stable angina pectoris (SAP) treated with everolimus-eluting stents (EES) or sirolimus-eluting stents (SES).
Methods and results: We performed a post hoc subgroup analysis of data from SORT OUT IV. Of 2,705 patients, 1,178 (43.5%) patients had ACS and were treated with EES (n=580) or SES (n=598), and 1,527 (56.5%) patients had SAP and were treated with EES (n=773) or SES (n=754). The primary composite endpoint was major adverse cardiac events (MACE): cardiac death, myocardial infarction (MI), stent thrombosis, or target vessel revascularisation within 18 months. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated for the endpoints.
At 18-month follow-up, patients with ACS had higher rates of MACE compared to patients with SAP (8.1% versus 6.7%; HR=1.23, 95% CI: 0.93-1.62). MACE did not differ significantly between ACS patients treated with EES or SES (7.3% versus 8.9%; HR=0.81, 95% CI: 0.54-1.22) nor between SAP patients treated with EES or SES (6.9% versus 6.5%; HR=1.05, 95% CI: 0.71-1.55).
Conclusions: EES and SES performed similarly with respect to MACE at 18-month follow-up in patients with ACS and SAP.
Introduction
Compared with bare metal stents (BMS), first-generation drug-eluting stents (DES) have reduced the need for repeat revascularisation1,2. However, safety concerns related to first-generation DESs have arisen, as they may lead to delayed healing and endothelial dysfunction of the stented arterial segment, increasing the risk of late thrombotic events3. Second-generation DESs were developed to improve the safety and efficacy of these devices4. Recent randomised trials with head-to-head comparisons of the first-generation paclitaxel-eluting TAXUSTM stent (PES) (Boston Scientific, Natick, MA, USA) with second-generation stents suggest that the newer DESs are more effective and are safer5-7.
The second-generation everolimus-eluting stent (EES) represents a potential step forward in DES technology, characterised by high deliverability and a tissue-gentle polymer coating8-10. Currently it is one of the most commonly used drug-eluting stents11. A meta-analysis suggested that the sirolimus-eluting stent (SES) is a more effective and safer first-generation device than the PES1. Randomised data comparing the EES with the SES are limited5,11-16. Therefore, direct head-to-head comparison of the EES and SES is of major clinical interest in defining the role of EES in the treatment of patients with coronary artery disease.
Patients with acute coronary syndromes (ACS) constitute an important high-risk group for acute and late adverse events12. In order to establish the long-term safety and efficacy of EES versus SES in ACS patients, we conducted a subgroup analysis of SORT OUT IV randomised clinical trial data, comparing the clinical outcome of EES versus SES in patients with ACS and with stable angina pectoris (SAP).
Methods
PATIENTS AND STUDY DESIGN
The SORT OUT IV trial was designed as a prospective, multicentre, all-comer, two-arm, randomised, non-inferiority study comparing the EES to the SES in treating atherosclerotic coronary artery lesions17. The study period was August 2007 to June 2009. The detailed study protocol is provided in the main publication18. Briefly, patients were eligible if they were at least 18 years old, had chronic stable coronary artery disease or acute coronary syndromes, and at least one coronary lesion with more than 50% diameter stenosis, requiring treatment with a DES. If multiple lesions were treated, the allocated study stent had to be used in all lesions. No restrictions were placed on number of treated lesions, number of treated vessels, or lesion length. Exclusion criteria were life expectancy of less than one year; allergy to aspirin, clopidogrel, sirolimus, or everolimus; participation in another randomised trial; or inability to provide written informed consent.
RANDOMISATION
Patients were enrolled by the investigators and randomly allocated to treatment groups after diagnostic coronary angiography and before the percutaneous coronary intervention. Block randomisation by centre (permuted blocks of random sizes [2/4/6]) was used to assign patients in a 1:1 ratio to receive the EES (XIENCE V®; Abbott Vascular, Redwood City, CA, USA; or PROMUSTM [Abbott’s privately-labelled XIENCE V everolimus-eluting coronary stent system distributed by Boston Scientific Corporation]) or the SES (Cypher Select Plus; Cordis, Johnson & Johnson, Warren, NJ, USA). The allocation sequence was computer-generated and stratified by gender and presence/absence of diabetes. Patients were assigned to treatment through an automated telephone allocation service. While operators were unblinded, the clinical events committee was masked to treatment assignment.
STUDY PROCEDURES
The EES was available in diameters of 2.25-4.00 mm and lengths of 8-28 mm. The SES was available in diameters of 2.25-3.50 mm and lengths of 8-33 mm. Stents were implanted according to standard techniques. Angiographic variables were visually estimated by the operators. Direct stenting without prior balloon dilation was allowed. Before or at the time of the procedure, patients received at least 75 mg of aspirin, a 600 mg loading dose of clopidogrel, and an unfractionated heparin dose (5,000 IU or 70-100 IU/kg).
Glycoprotein IIb/IIIa inhibitors were used at the operator’s discretion. Recommended post-procedure dual antiplatelet regimes were 75 mg aspirin daily for life and 75 mg clopidogrel daily for one year.
ENDPOINTS
Definition of endpoints has been provided elsewhere17,18. The primary endpoint of this substudy was a composite of safety (cardiac death, myocardial infarction, definite stent thrombosis) and efficacy (clinically indicated target vessel revascularisation) parameters within 18 months of stent implantation. Individual components of the primary endpoint comprised the secondary endpoints: cardiac death, myocardial infarction, definite stent thrombosis, clinically indicated target vessel/lesion revascularisation, probable, possible, and overall stent thrombosis according to the Academic Research Consortium (ARC) definition19, and device failure (defined as inability to implant the assigned study stent in the target lesions).
COMORBIDITY
For all patients, we obtained data on all hospital diagnoses from the Danish National Registry of Patients, covering all Danish hospitals, from 1977 until the implantation date20. We then computed Charlson Comorbidity Index scores, which encompass 19 major disease categories, including diabetes mellitus, heart failure, cerebrovascular diseases and cancer21.
Clinical event detection
Clinically driven event detection was used to avoid study-induced reinterventions. Data on mortality, hospital admissions, coronary angiography, repeat percutaneous coronary intervention, and coronary bypass surgery were obtained for all randomly allocated patients from the following national Danish administrative and healthcare registries: the Civil Registration System22; the Western Denmark Heart Registry23,24; the Danish National Registry of Patients25, which maintains records on all hospitalisations in Denmark; and the Danish Registry of Causes of Death26. An independent clinical events committee reviewed all endpoints and source documents to adjudicate causes of death, reasons for hospitalisation, and diagnoses of myocardial infarction. Cine films were reviewed by the committee to classify stent thrombosis and target vessel revascularisation (following percutaneous coronary intervention or coronary artery bypass grafting).
The Danish National Health Service provides universal tax-supported health care, guaranteeing residents free access to general practitioners and hospitals. The Danish Civil Registration System has kept electronic records on gender, birth date, residence, emigration date, and vital status changes since 196822, with daily updates; the 10-digit civil registration number assigned at birth and used in all registries allows accurate record linkage. The Civil Registration System provided vital status data for our study participants and minimised loss to follow-up. The National Registry of Causes of Death and the Danish National Registry of Patients provided information on causes of death and diagnoses assigned by the treating physician during hospitalisations (coded according to the International Classification of Diseases, 10th revision [ICD-10])25.
Statistical analysis
Data are presented as mean±SD or median and interquartile range for continuous data or as counts. Distributions of continuous variables were compared between study groups using the two-sample t-test (or Cochran test for cases with unequal variance) or the Mann-Whitney U test, depending on whether the data followed a normal distribution. Distributions of categorical variables were compared using the χ2 test. In analyses of every endpoint, follow-up continued until the date of an endpoint event, death, emigration, or 18 months after stent implantation, whichever came first. Survival curves were constructed based on cumulated incidences, accounting for death as a competing risk27. Hazard ratios were computed using Cox proportional hazards regression analysis. Patients treated with the SES were used as the reference group for subgroup analyses. Hazard ratios were computed for major adverse cardiac events at 18-month follow-up. The intention-to-treat principle was used in all analyses. A two-sided p-value of less than 0.05 was considered to indicate statistical significance. Analyses were conducted using SAS software version 9.2 (SAS Institute Inc., Cary, NC, USA). This trial is registered with ClinicalTrials.gov, number NCT00552877.
Results
PATIENTS AND ENROLMENT
The SORT OUT IV trial randomised 2,705 patients with ACS (EES=580, SES=598) or SAP (EES=773, SES=754). In the ACS group, 122 (21%) and 145 (24%) patients were treated for ST-segment elevation myocardial infarction in the EES and SES groups, respectively. One patient was lost to follow-up on day 187 because of emigration (this patient had SAP at the time of the index procedure, which was considered a non-event in the primary endpoint analysis).
Baseline patient characteristics differed between the ACS and SAP groups (Table 1).
Compared to patients with SAP, patients with ACS were slightly younger (62.8±11.4 years vs. 65.1±10.4 years, p=0.0001), more often active smokers, and had lower rates of diabetes mellitus, hypertension, hypercholesterolaemia, and previous revascularisation. Prevalence of previous MI did not differ between the two indication groups. The comorbidity index score was significantly lower in patients treated for ACS compared to patients treated for SAP (Table 1).
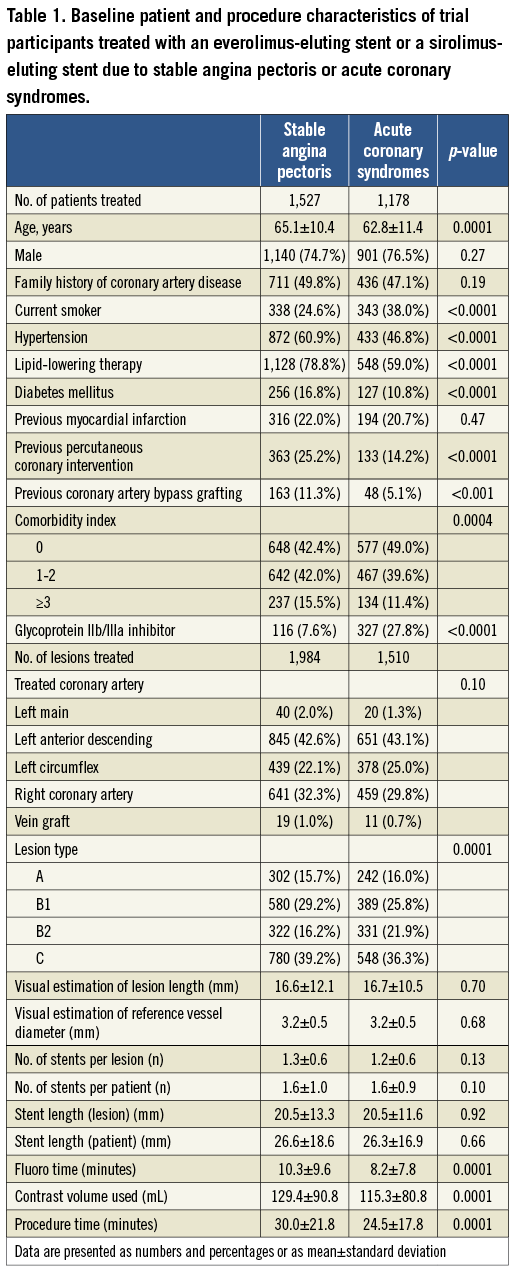
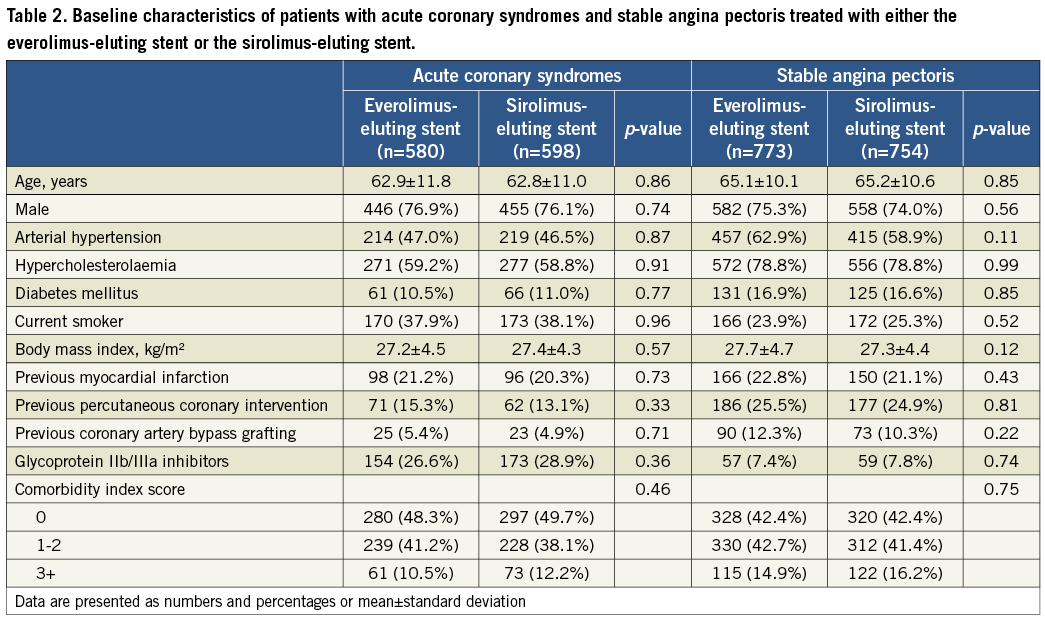
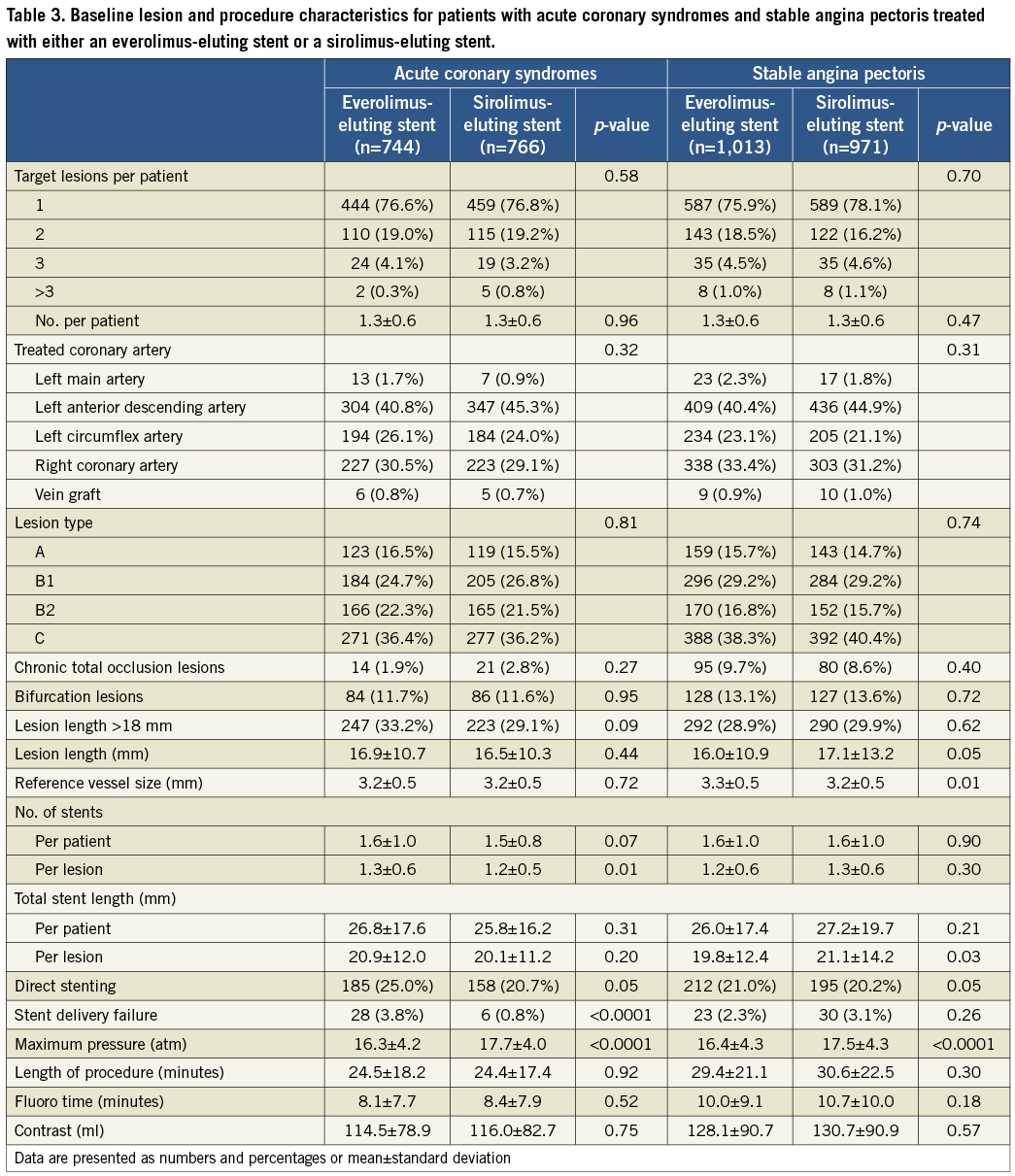
Baseline parameters were well balanced in both the EES and SES treatment subgroups of the ACS and SAP groups (Table 2). EES-treated ACS patients had significantly more stents per patient (1.3±0.6 vs. 1.2±0.5, p=0.01), and stents per lesion (1.3±0.6 vs. 1.2±0.5, p=0.01), compared with the SES-treated ACS patients. EES-treated ACS patients more often had direct stenting (25.0% vs. 20.7%, p=0.05). EES-treated SAP patients had shorter lesion lengths, larger reference vessel sizes, shorter stent lengths per lesion, and lower balloon pressure applied periprocedurally, compared to SES-treated SAP patients (Table 3).
CLINICAL OUTCOMES
CLINICAL OUTCOMES ACCORDING TO INDICATION
At 18 months, MACE did not differ significantly among ACS patients compared to SAP patients (8.1% vs. 6.7%; HR=1.23, 95% CI: 0.93-1.62) (Figure 1).
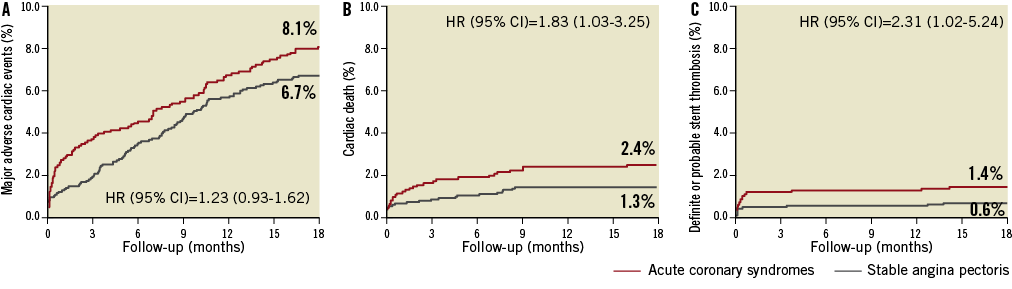
Figure 1. Time-to-event curves for major adverse cardiac events. Major adverse cardiac events are the composite of cardiac death, myocardial infarction, definite stent thrombosis, and target vessel revascularisation. Major adverse cardiac events (A), cardiac death (B), definite or probable stent thrombosis (C) in patients treated with everolimus-eluting stents or sirolimus-eluting stents due to acute coronary syndromes or stable angina pectoris.
When we tested for interaction between clinical presentation and type of drug-eluting stent, we found no significant interaction for the composite endpoint MACE (p=1.00).
Rates of cardiac death (2.4% vs. 1.3%; HR=1.83, 95% CI: 1.03-3.25) and definite and probable stent thrombosis (1.4% vs. 0.6%; HR=2.31, 95% CI: 1.02-5.24) were significantly higher among patients with ACS compared to patients with SAP, while rates of MI, TVR, and TLR did not differ significantly between the two groups. Of the 48 cardiac deaths observed in the total study population, nine (18.8%) occurred in-hospital (EES n=5, and SES n=4). Among patients with ACS, MACE did not differ significantly between STEMI patients and UAP/NSTEMI patients (7.1% vs. 8.4%; HR=0.85, 95% CI: 0.52-1.41).
CLINICAL OUTCOMES BY STENT TYPE
In patients with ACS, treatment with EES compared to SES did not affect MACE significantly at 18 months (7.3% vs. 8.9%; HR=0.81, 95% CI: 0.54-1.21) (Figure 2).
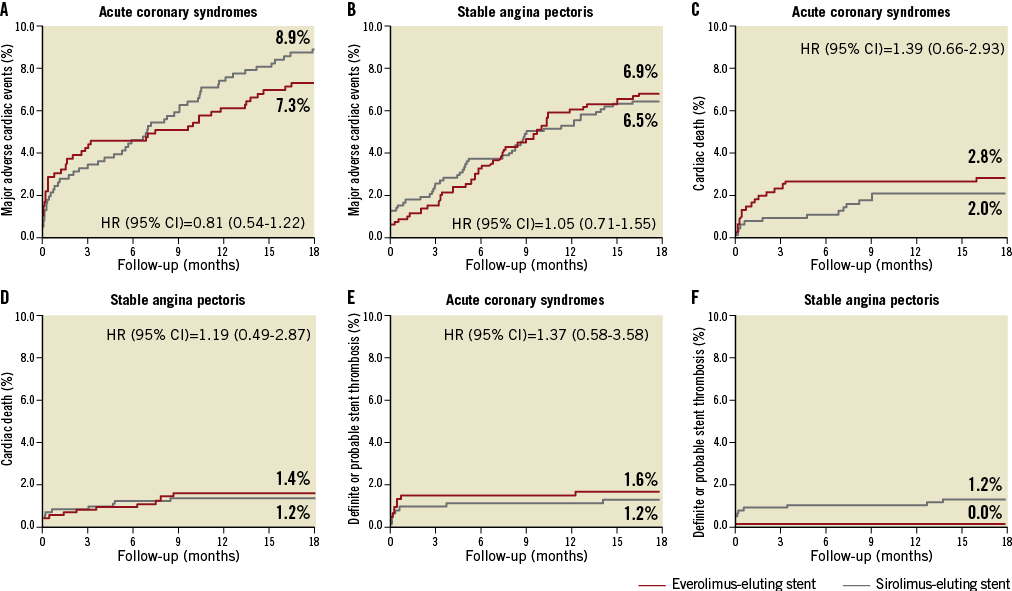
Figure 2. Time-to-event curves for major adverse cardiac events. Major adverse cardiac events are a composite of cardiac death, myocardial infarction, definite stent thrombosis, and target vessel revascularisation. MACE (A: stable angina; B: acute coronary syndromes), cardiac death (C: stable angina; D: acute coronary syndromes), definite or probable stent thrombosis (E: stable angina; F: acute coronary syndromes) in patients treated with everolimus-eluting stents versus patients treated with sirolimus-eluting stents.
The other endpoints also did not differ significantly between the two treatment subgroups.
In patients with SAP, treatment with EES compared to SES did not influence the prevalence of the composite endpoint (6.9% vs. 6.5%; HR=1.05, 95% CI: 0.71-1.55) (Figure 2).
Overall, there were no significant differences in rates of stent thrombosis in SES versus EES patients with ACS or SAP (Table 4 and Figure 2). Definite stent thromboses were not observed in SAP patients treated with EES, while eight cases of definite stent thrombosis were registered among SAP patients treated with SES. Among SAP patients treated with SES, most definite stent thromboses occurred within 30 days of the index procedure. There was only one case of very late definite stent thrombosis.
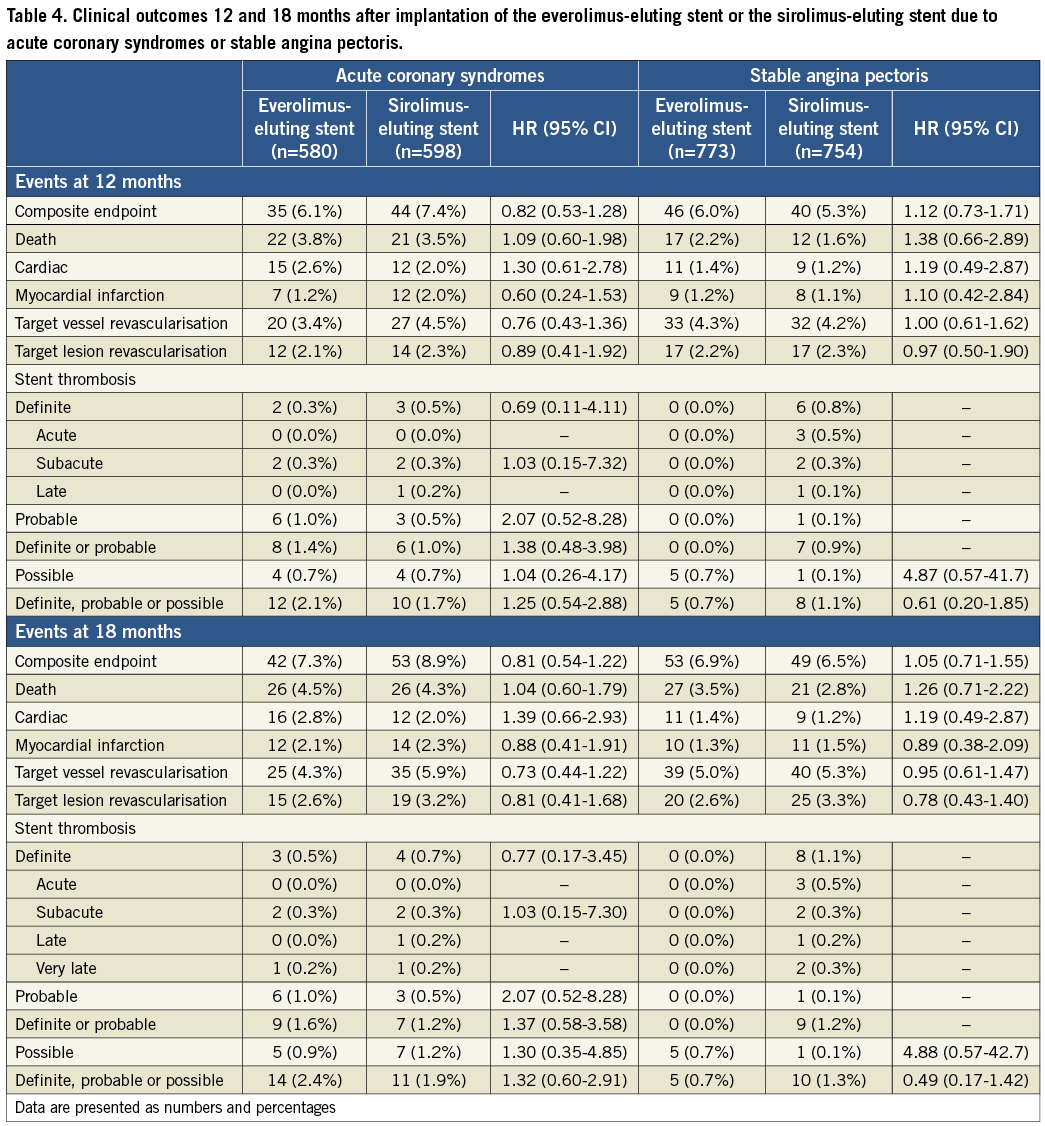
An analysis of outcomes according to type of ACS (ST-segment elevation myocardial infarction [STEMI] versus non-ST-segment elevation myocardial infarction [NSTEMI]) is summarised in Table 5. Outcomes did not differ significantly by type of ACS, or by type of stent implanted in STEMI patients.
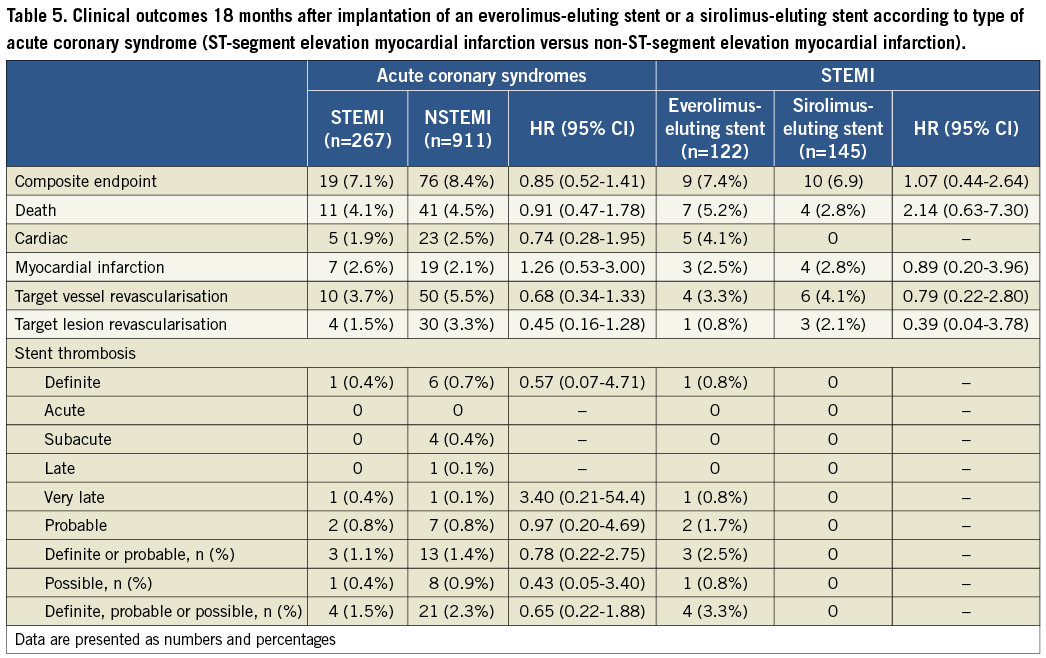
A stratified analysis for major subgroups with respect to the primary endpoint in the ACS patients was assessed in a forest plot (Figure 3).
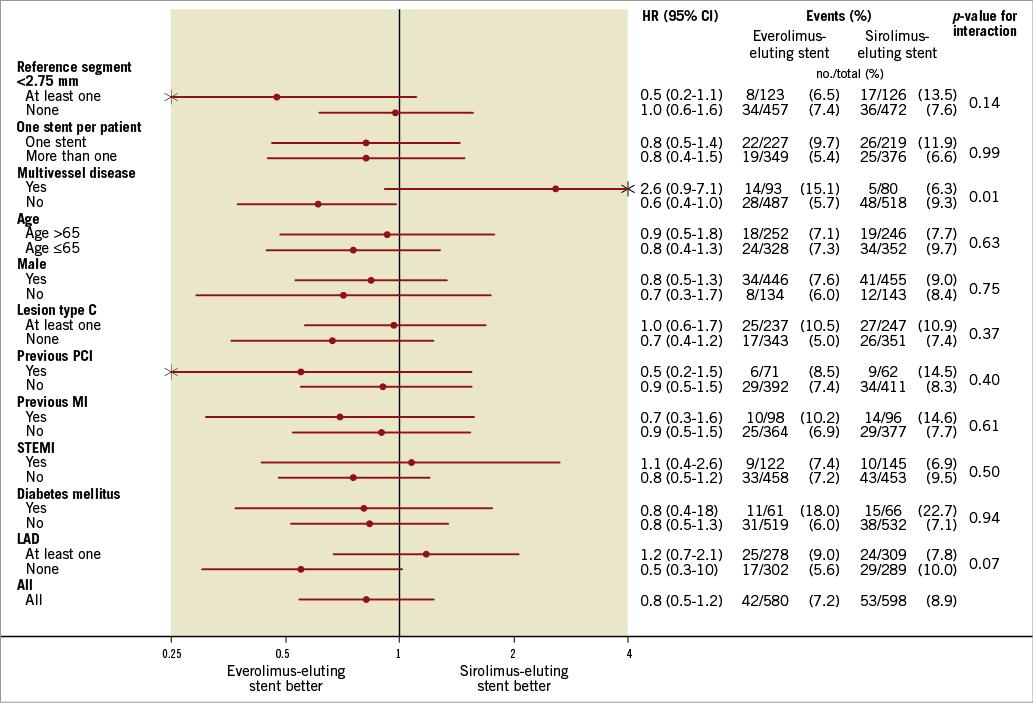
Figure 3. Forest plot summarising a stratified analysis for major subgroups with respect to the primary endpoint in ACS patients.
Discussion
This substudy of the SORT OUT IV trial focused on safety and efficacy of EES versus SES in patients with ACS or SAP. The main findings may be summarised as follows: (1) patients with ACS had a non-significantly higher MACE rate than patients with SAP; (2) the MACE rate did not differ significantly among EES and SES treated patients with ACS or SAP, respectively; and (3) definite stent thromboses were not observed in SAP patients treated with EES at 18-month follow-up.
Despite the improved efficacy of the most recent DESs, ACS continues to be a determinant of impaired prognostic outcome28,29. This is shown in the results of the present study, in which patients with ACS had a higher MACE rate than patients with SAP. Information about potential differences in outcome after treatment with a first versus second-generation DES in the high-risk ACS group is limited and has not previously been studied in a randomised trial. Planer et al30 recently compared clinical outcomes of treatment with EES versus PES in patients with ACS and SAP by pooling results from the “Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease” (SPIRIT)31-34 and “Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice” (COMPARE)35 trials. In agreement with our results, they found that treatment of ACS patients was associated with a higher rate of MACE at two years. In the pooled analysis of data from the SPIRIT and COMPARE trials30, treatment with EES provided enhanced safety and efficacy compared with PES amongst both ACS and SAP patients. In our study, we saw a numerically, but not statistically significant, difference in MACE rates between subgroups receiving EES versus SES. The lack of significance may be partly explained by EES being superior to SES11, and partly by lack of power in this subgroup analysis.
Kalesan et al36 recently compared the long-term clinical outcome of EES versus SES in patients with ACS. Propensity-score matching was used, and clinical outcome was compared among 705 matched pairs of ACS patients treated with EES or SES. They found that unrestricted use of EES was associated with an improved longer-term clinical outcome compared with SES, mainly because of a lower TVR rate after EES implantation. This is in line with our results, where MACE and TVR events were numerically lower in the EES subgroup of ACS patients. It is possible that there will be significant differences in clinical outcomes among ACS patients treated with EES versus those treated with SES during long-term follow-up.
Regarding the stratified analysis for subgroups with respect to the primary endpoint in the ACS patients (forest plot, Figure 3), we found a significant p-value for interaction in patients with multivessel disease. However, we do not have an explanation for that, and this significant result could have been found by chance.
Two recent meta-analyses including large, randomised trials comparing EES versus SES in patients with coronary artery disease found no significant differences in efficacy and safety clinical outcomes5,11. Also, recent randomised trials comparing EES versus SES in other high-risk groups reported clinical outcome results comparable with those in the present study12,14,16.
Patient-related composite and stent-related composite outcomes were assessed in patients treated with the EES versus the zotarolimus-eluting Resolute stent (Re-ZES; Medtronic, Minneapolis, MN, USA) in the “Unrestricted use of two new generation drug-eluting coronary stents: two-year patient-related versus stent-related outcomes from the RESOLUTE All Comers trial”37. Similar safety and efficacy outcomes were sustained between these two new-generation DESs at two-year follow-up in a mostly complex population of patients with ACS and SAP. Also, in “A randomised controlled trial in second-generation zotarolimus-eluting Resolute stents versus everolimus-eluting XIENCE V stents in real-world patients (the TWENTE trial)”38, the Re-ZES was non-inferior to the XIENCE V EES with target vessel failure in a patient population where half of the patients were treated due to non-STEMI ACS.
ACS patients treated with PCI and DES represent patients with an additional risk of morbidity and mortality, compared to patients with stable coronary artery disease29,39. Intervention in a pro-thrombotic, inflammatory environment may result in compromised vessel healing, delayed re-endothelialisation, and enhanced agonist-induced platelet aggregation28,40-42.
Overall, the risk of stent thrombosis did not differ significantly between ACS patients treated with EES versus SES. However, results seemed to favour the EES in treatment of SAP patients, as we found no definite stent thromboses within 18 months in this subgroup. Different mechanisms of action of drugs or polymers, in terms of inhibiting neointimal growth and vascular healing, may explain the disparity in risk of stent thrombosis in different clinical syndromes after EES and SES treatment. The mechanisms underlying a reduced risk of stent thrombosis with EES remain somewhat unclarified, but may be related to factors such as reduced strut thickness (81 µm versus 140 µm) resulting in a more rapid, complete and homogeneous endothelialisation, a thinner biocompatible polymer (7.6 µm versus 12.6 µm) potentially reducing the risk of vascular hypersensitivity reactions, and a lower dose of the antiproliferative drug36,43. Also, interactions between stent platform and lesion characteristics might be contributing factors28,29.
Due to an overall low frequency of stent thrombosis, large sample sizes are needed to estimate treatment differences between stents accurately. Palmerini et al44 recently assessed this clinical issue by providing a meta-analysis, including randomised trials comparing different drug-eluting stents or drug-eluting with bare metal stents. Using the ARC definition19, the EESs were associated with a significant reduction in definite stent thromboses compared with the BMSs at one-year follow-up. Additionally, the EESs were associated with a lower risk of definite stent thromboses compared with the PESs, the SESs, the phosphorylcholine polymer-based zotarolimus-eluting stents (PC-ZESs), and the Re-ZESs.
Also, a recent large-scale cohort study, provided by Räber et al45, assessed and compared the risk of very late stent thrombosis in 12,339 SES-, PES-, and EES-treated patients, where approximately 50-60% of the patients in the three stent subgroups were treated due to ACS. During follow-up of up to four years, they found that the EES was associated with a lower risk of very late stent thrombosis compared with early-generation drug-eluting stents, supporting the unrestricted use of the EES in a broad patient population.
The TLR rate was numerically lower in ACS and SAP patients treated with EES compared to those treated with SES in the present study, but the difference was not statistically significant. These data concur with recently published results from the “Biodegradable polymer versus permanent polymer drug-eluting stents and everolimus- versus sirolimus-eluting stents in patients with coronary artery disease” (ISAR-TEST 4) trial12, in which the EES and the SES showed similar three-year clinical efficacy. Also the EXCELLENT trial16 had similar findings regarding TLR when the EES and the SES were compared at 12 months.
Limitations
Although both treatment groups were recommended dual antiplatelet therapy for the same duration after stenting, complete data confirming actual duration are not available.
Also, longer follow-up is needed to follow and confirm the clinical outcomes of these drug-eluting stents in different clinical settings/indication subgroups.
Another concern is that the operators were not blinded to the stent type implanted; however, the clinical events committee was blinded regarding the stent type deployed.
Our findings showed fewer events, particularly fewer myocardial infarctions, than reported in other randomised trials (COMPARE, RESOLUTE, LEADERS). This difference can partly be explained by procedure-related myocardial infarction, which was not a part of the primary endpoint in the SORT OUT IV trial. In the LEADERS trial, the myocardial infarction rate increased 0.5% and 0.6%, respectively, from 30 days (biolimus-eluting stent=4.9%, and sirolimus-eluting stent=4.1%) to nine months post-implantation (biolimus-eluting stent=5.7%, and sirolimus-eluting stent=4.6%), indicating that the majority of myocardial infarctions were early, and predominantly related to stent implantation.
The SORT OUT IV trial was an all-comers trial; however, as in other all-comers trials, not all eligible patients were enrolled. Eligible non-randomised patients were older and were more often hospitalised with STEMI. This may influence an application to a larger STEMI population.
However, patient care complied with normal clinical practice, i.e., follow-up during a hospital outpatient visit after one to three months. We believe that this approach to clinically driven event detection combined with a randomised all-comers trial design allowed us to assess the efficacy of different percutaneous coronary interventions in a context reflecting everyday clinical practice during the study period.
This is a subgroup analysis of the SORT OUT IV trial and the size of the subgroups was not a priori powered for comparison. However, the large sample size and randomised design of our study provided sufficient power to indicate that there are no clinically important differences between EES and SES on MACE at 18-month follow-up in ACS and SAP patients.
Conclusion
EES and SES appear similar with respect to MACE at 18 months in patients with ACS and SAP. No definite stent thromboses were seen in SAP patients treated with EES.
Conflict of interest statement
L. O. Jensen has received honoraria from Abbott Vascular and Cordis (CEC member). P. Thayssen, J. Ravkilde, L. Thuesen, and J. F. Lassen have received unrestricted grants from Abbott Vascular and Cordis to their institutions. L. Thuesen and J. F. Lassen have received unrestricted grants from Boston Scientific to their institution. H. H. Tilsted has received travel grants from Abbott Vascular and Cordis. M. Maeng has received travel grants from Cordis, Medtronic and Boston Scientific. J. Ravkilde has received honoraria from Abbott Vascular. L. Thuesen has received honoraria from Abbott Vascular, Cordis, and Boston Scientific. J. F. Lassen has received speaking honoraria from Abbott Vascular, Cordis, Medtronic, Eli Lilly, Boston Scientific, and AstraZeneca. The other authors have no conflicts of interest to declare.

