Abstract
Aims: To evaluate the clinical outcomes of sirolimus-eluting stent (SES) versus bare metal stent (BMS) implantation in patients with ST-segment elevation myocardial infarction (STEMI) at long-term follow-up.
Methods and results: After five years, 310 STEMI patients randomly assigned to implantation of either SES or BMS, were compared. Survival rates were comparable between groups (SES 94.3% vs. BMS 92.8%, p=0.57), as were the rates of reinfarction (10.6% vs. 13.7%, p=0.40), freedom of death/re-MI (84.4% vs. 79.8%, p=0.29) and target vessel failure (14.9% vs. 21.7%, p=0.11). Likewise, rates of overall stent thrombosis (ST) (5.4% vs. 2.7%, p=0.28) and very late ST (4.1% vs. 0.7%, p=0.07) did not significantly differ between the SES- and BMS-group. In 184 patients with IVUS data, definite and definite/probable VLST was more common in those with late stent malapposition versus those without late stent malapposition (4.3% and 6.6% vs. no events [p=0.018 and p=0.004], respectively). The cumulative incidences of target vessel and target lesion revascularisation (TVR and TLR) were not significantly lower in the SES-group (11.2% vs. 17.9%, p=0.09 and 7.2% vs. 12.9%, p=0.08), as was the rate of clinically driven TLR (6.6% vs. 9.5%, p=0.30).
Conclusions: SES implantation was neither associated with increased rates of major adverse cardiac events, nor with a reduction in re-intervention, compared to implantation of a BMS in patients with STEMI after five years. However, a trend of more very late stent thrombosis was observed after SES implantation (ISRCTN62825862).
Abbreviations
STEMI: ST-elevation myocardial infarction
PCI: percutaneous coronary intervention
DES: drug-eluting stent
BMS: bare metal stent
SES: sirolimus-eluting stent
PES: paclitaxel-eluting stent
(A)MI: (acute) myocardial infarction
CABG: coronary artery bypass grafting
TVR: target vessel revascularisation
TLR: target lesion revascularisation
TVF: target vessel failure
ST: stent thrombosis
VLST: very late stent thrombosis
TIMI: thrombolysis in myocardial infarction
IVUS: intravascular ultrasound
Introduction
In the treatment of ST-segment elevation myocardial infarction (STEMI), primary percutaneous coronary intervention (PCI) is the treatment of choice to achieve coronary reperfusion1. With the development of drug-eluting stents (DES), the short- and mid-term prognosis for this specific group of patients has improved in terms of repeat target vessel revascularisation as compared to bare metal stents (BMS). However, the use of DES did not result in lower rates of death, recurrent myocardial infarction or stent thrombosis2.
Since the first expression of concern about the safety of DES3-6, these stents remain a topic of debate, especially in the treatment of patients with off-label indications, like STEMI. Despite reassuring outcomes of most studies, there is still no definite consensus on this matter since the question has been raised that there may be an increased risk of late events in these patients7. The few randomised controlled trials that actually have very long-term follow-up data available8-11, showed contradictory results for efficacy and safety, in particular concerning very late stent thrombosis (i.e., stent thrombosis beyond one year after the index procedure). Therefore, efficacy and safety outcomes of DES versus BMS were evaluated in patients with STEMI five years after participation in the MISSION! Intervention Study.
The methods were previously described in detail in an earlier publication and will only be briefly touched on hereafter12.
Design
The MISSION! Intervention study was a prospective randomised controlled trial (ISRCTN62825862) that aimed to compare the performance of sirolimus-eluting stents (SES) versus BMS for the treatment of acute myocardial infarction (AMI). It was a single-centre, single-blind study, designed to evaluate the angiographic and intravascular ultrasound outcomes (IVUS) at nine months and clinical outcomes at 12 months after stent implantation. Follow-up angiography was performed at nine months in the majority of the study population (81.6% in BMS-group and 82.9% in SES-group) and at three years in a minority (32.2% in BMS-group and 40.5% in SES-group), based on the presence of informed consent given by patients.
The study protocol was approved by the institutional ethics committee, and written informed consent was obtained from all patients before enrolment, and separately for the 9-months and 3-year coronary angiography. Patients were included from February 2004 to October 2006. The present study evaluated clinical outcomes up to five years after the index procedure.
Subjects
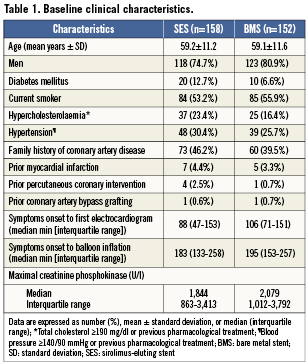
Patients were eligible if symptoms of AMI started <9 hours before arrival at the catheterisation laboratory and the electrocardiogram demonstrated a STEMI. Criteria for exclusion were age ≤18 years or ≥80 years, left main stenosis of ≥50%, triple vessel disease (defined as ≥50% stenosis in ≥3 major epicardial branches), previous PCI or coronary artery bypass grafting (CABG) of the infarct-related artery, thrombolytic therapy for the index infarction, target lesion length of >24 mm, a visually estimated target vessel reference diameter <2.25 mm or >3.75 mm, the need for mechanical ventilation, known renal failure, patients with a life expectancy <12months and those with a contraindication for the use of aspirin, clopidogrel, heparin or abciximab. Randomisation to treatment with a BMS (Vision; Guidant Corp., Indianapolis, IN, USA) or SES (Cypher; Cordis Corp., Miami Lakes, FL, USA) was performed in a 1:1 ratio.
Study procedure
Before the index procedure, all patients received aspirin 300mg, clopidogrel 300 to 600mg and an intravenous bolus of abciximab 25µg/kg with subsequent continuous infusion of 10µg/kg/min for 12 hours. At the start of the procedure, heparin 5,000 IU was given. Lesions were treated according to current interventional practice, with direct stenting allowed. IVUS imaging at 9-month follow-up was performed with motorised pullback (0.5mm/s), starting at least 10 mm distal to the stent and ending at the coronary ostium using a2.9 Fr 20-MHz catheter and a dedicated IVUS console (Eagle Eye; Volcano Corp., Rancho Cordova, CA, USA) and was preceded by 200 to 300µg of intracoronary nitroglycerine.
Follow-up and data collection
Patients were seen at the outpatient clinic at 30 days, 3, 6 and 12 months according to the MISSION! AMI care program13. Both treatment groups received antiplatelet therapy for equal treatment duration, consisting of lifelong aspirin 100 mg daily and clopidogrel 75mg daily for the first 12 months. Furthermore, patients were treated with beta blocking agents, statins and angiotensin-converting enzyme inhibitors for at least one year.
After the first year of follow-up, patients were monitored according to protocol with outpatient clinic visits and/or telephone inquiry. Follow-up data were prospectively collected in the electronic patient file (EPD Vision version 8.7.0.1). If necessary, cardiologists from other clinics and general practitioners were contacted for further information. From patients who died during follow-up, hospital records were reviewed and the cause of death was ascertained.
IVUS analysis
IVUS images were analysed offline, using quantitative IVUS analysis software (QCU-CMS 4.14; Medis, Leiden, The Netherlands). Analyses were performed by two experienced analysts blinded for the assigned treatment. Stent malapposition was defined as separation of at least one stent strut from the intimal surface that was not overlapping a side branch, and had IVUS evidence of blood speckles behind the strut14.
Study endpoints
The endpoints of the present study were death, recurrent myocardial infarction (re-MI), freedom of death/re-MI, target vessel revascularisation (TVR), (clinically driven) target lesion revascularisation (TLR), target vessel failure (TVF) and stent thrombosis (ST). All deaths were defined as cardiac, unless unmistakably proved non-cardiac. Myocardial infarction during follow-up was defined as a troponin-T above the upper limit in the presence of symptoms or PCI, a rise of troponin-T of >0.15µg/l after coronary artery bypass grafting or a re-rise of troponin-T of >25% after recent myocardial infarction in the presence of symptoms or PCI. All infarctions were categorised as spontaneous or procedure related (non-index procedure). Freedom of death/re-MI included death from any cause and re-MI according to the above definition. TVR and TLR were defined as any revascularisation procedure of the target vessel or lesion with lesion area defined as 5 mm distally to the stent up to 5mm proximally to the stent. Clinically driven TLR was defined as repeated TLR (with ≥50% diameter stenosis), driven by clinical symptoms at rest in conjunction with electrocardiographic evidence of ischaemia or (silent) ischaemia demonstrated by functional tests. TVF was the composite of cardiac death, nonfatal re-MI that was attributable to the target vessel, or any revascularisation procedure of the target vessel. All events were considered to be culprit vessel related unless unequivocally attributable to a non-culprit vessel. Stent thrombosis was defined as definite, probable, or possible (the composite of these being any ST), according to the Academic Research Consortium definition. These groups were further subdivided into acute (≤1 day), subacute (>1 day to ≤1 month), late (>1month to ≤1 year), and very late (>1 year).
Statistical design and analysis
Design and sample-size calculations were performed for the original purpose of the study only12. Analyses were conducted according to the intention-to-treat principle. Continuous variables were compared between the treatment groups with a t-test or, in case of a non-Gaussian distribution, with a nonparametric test. The Kaplan-Meier method was used to estimate cumulative incidence rates of all endpoints at five years. Event rates were compared between treatment groups with the log-rank test. Hazard ratios and 95% confidence intervals were estimated with the use of Cox proportional-hazard models with stent type as the only covariate. As a measure of clinical implications, numbers needed to treat and to harm were calculated for clinically driven target lesion revascularisation and stent thrombosis, respectively. All p-values were 2-sided, and ap-value <0.05 was considered to be statistically significant. All analyses were conducted with SPSS version 17.0.1 statistical analysis software (SPSS Inc., Chicago, Il, USA).
Results
Patients
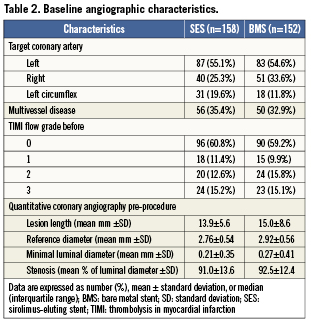
Between February 2004 and October 2006, 575 patients were screened of whom 316 patients were enrolled in the study. Six patients were excluded after randomisation because the assigned study stent was not available. Finally, 310 patients were included in the analysis; 158 patients were assigned to treatment with aSES, and 152 patients to treatment with a BMS (Figure1). Baseline characteristics were comparable between the two groups (Tables1 and 2), except for a larger reference diameter in the BMS-group.
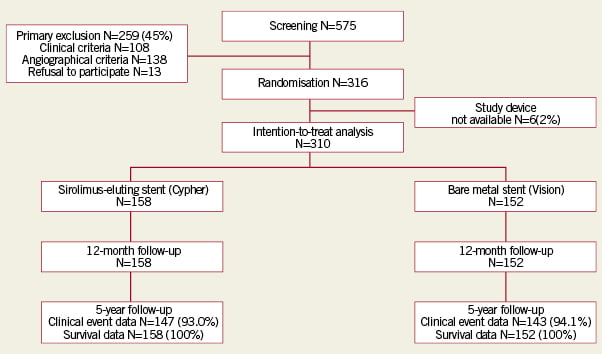
Figure 1. Patient flowchart. Inclusion and follow-up of patients
Follow-up and medication
Complete clinical data were available in 93.0% and 94.1% of the SES- and BMS-group, respectively. Survival status was available for all patients (Figure1). Clinical data of patients considered to be lost to follow-up were included until the last follow-up date. Clopidogrel was used for at least 12 months by 93% of the patients in the SES-group and 96.1% (p=0.24) of patients in the BMS-group. Prescription was extended beyond one year or restarted when clinically indicated (after a recurrent ischaemic event or repeat PCI).
Clinical outcomes
Adverse events up to five year are listed in Table3. As reported previously12, clinical outcomes at 12 months of follow-up showed asignificant difference in the occurrence of revascularisation procedures, TVR, TLR and TVF. However, there was no difference in rates of death, re-MI or ST. At three years of follow-up15 previously reported differences in TVR, TLR and TVF at 12 month follow-up were no longer significant. Rates of death, re-MI and ST remained comparable between treatment groups. At five years, survival rate in the SES-group was 94.3% versus 92.8% in the BMS-group (p=0.57, Figure 2). Cardiac cause of death was present in 10 patients; five in each treatment group. Re-MI occurred in 16 (10.6%) SES patients and 20 (13.7%) BMS patients (p=0.40), of which seven (4.5%) and 12 (8.1%) were related to a recurrent PCI procedure, respectively (p=0.20). Spontaneous re-MI occurred in nine (6.1%) SES patients and eight (5.6%) BMS patients (p=0.88). Freedom of death and re-MI was present in 84.4% of patients treated with SES and in 79.8% of patients treated with BMS (p=0.29, Figure 2).
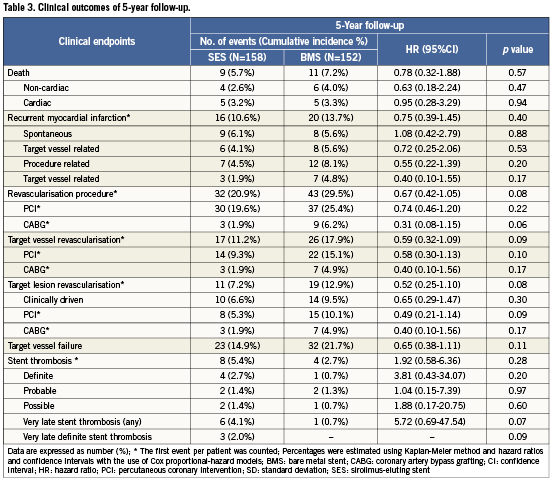
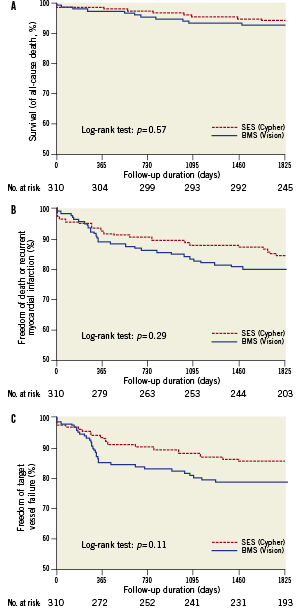
Figure 2. Safety of sirolimus-eluting stents (SES) versus bare metal stents (BMS). Kaplan-Meier time-to-event curves for A. survival (from death of any cause), B. survival free of death of any cause/re-MI and C. survival free of target vessel failure (composite of cardiac death, nonfatal re-MI attributable to the target vessel or any revascularisation procedure of the target vessel).
Of the patients treated with SES, 20.9% underwent a recurrent revascularisation procedure versus 29.5% of those treated with BMS (p=0.08). TVR occurred in 11.2% and 17.9% of SES- and BMS-treated patients, respectively (p=0.09). Recurrent TLR occurred in 7.2% of the SES-treated patients versus 12.9% of the BMS-treated patients (p=0.08), of which 6.6% vs. 9.5% were clinically driven (p=0.30), respectively. The number needed to treat (NNT) for SES compared to BMS to prevent one clinically driven TLR was 34.5 after a follow-up duration of five years.
Survival free of TVF occurred in 85.1% of the SES-group versus 78.3% of the BMS-group (p=0.11, Figure 2).
Definite ST was found in four (2.7%) patients treated with SES versus one (0.7%) in those treated with BMS (p=0.20). Rates of any ST (definite, probable and possible) were 5.4% and 2.7% in the SES- and BMS-group, respectively (p=0.28, Figure 3). The composite of definite or probable ST occurred in 4.0% of the SES-group and 2.0% of the BMS-group (p=0.35, Figure 3). The incidence of any very late ST (VLST) was 4.1% in the group treated with SES versus 0.7% in the group treated with BMS (p=0.07). As compared with BMS, the number needed to harm (NNH) after five years for SES was 37 for any ST, 50 for definite ST and 29.4 for VLST.
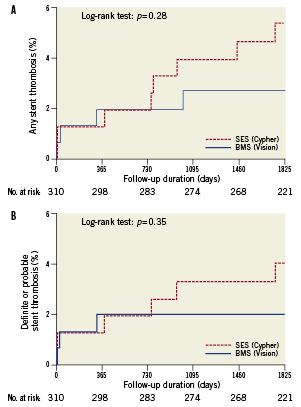
Figure 3. Stent thrombosis after sirolimus-eluting stent (SES) versus bare metal stent (BMS) implantation. Kaplan-Meier time-to-event curves, for A. any stent thrombosis (definite, probable and possible) and B. composite of definite or probable stent thrombosis.
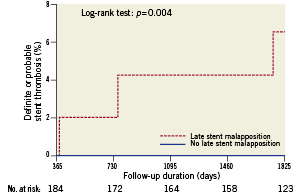
Figure 4. Very late stent thrombosis in patients with versus without late stent malapposition at 9-month routine follow-up IVUS. Kaplan-Meier time-to-event curve for the composite of definite or probable very late stent thrombosis.
IVUS outcomes
Routine 9-month follow-up IVUS data were available in 184 out of all 310 patients (60%). In 49 patients (26.6%) late stent malapposition was present.
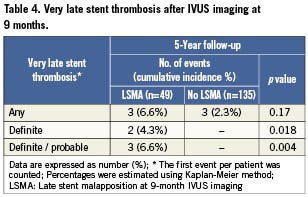
Of all patients who underwent IVUS imaging, definite VLST was significantly increased in those with late stent malapposition (4.3%) versus those without late stent malapposition (no events, p=0.018), as well as the composite of definite or probable VLST (6.6% versus no events, p=0.004) (Table 4).
Discussion
In this single-centre, randomised controlled trial, the long-term clinical performance of SES and BMS was evaluated in the treatment of STEMI patients. Major findings were: 1) There were no differences in terms of death, re-MI or target vessel failure after five years of follow-up. 2) Rates of overall and definite ST were comparable, however, atrend of more VLST was observed after SES implantation. 3) Of all patients who underwent 9-months IVUS imaging, VLST was more common among those with late stent malapposition compared to those without. 4) Though a non-significant trend of less re-interventions among SES-treated patients was observed, there was no evidence for areduction in clinically driven target lesion revascularisation.
Efficacy of DES
Implantation of DES has been demonstrated to be superior to BMS in terms of reduced need for TVR in patients undergoing elective PCI16, which led to regulatory approval for the use of DES in this setting. Since then, the performance of drug-eluting stents has been assessed for all sorts of lesion types17,18. A number of randomised trials evaluated the performance of DES compared to BMS exclusively in patients presenting with STEMI (e.g., the large HORIZONS-AMI trial19,20) and reported decreased rates of re-intervention as well. However, only a few trials have actually published long-term follow-up results so far (i.e., four years or more after the index procedure)8-11.
In line with the results of previous publications concerning stent implantation in the elective setting are the 4-year results of the TYPHOON9 and PASEO trial8, and 5-year results of the STRATEGY10, that all demonstrated a reduction in TLR or TVR with the use of DES compared to BMS in STEMI patients. In contrast, the PASSION trial11 did not report reduced rates of TLR in STEMI patients treated with a paclitaxel-eluting stent (PES) after five years of follow-up. Though differences between treatment groups were not significant in the present trial, a trend towards less re-intervention among SES-treated patients was observed. This is mainly attributed to the preservation of the benefit that was acquired during the first year of follow-up. Thereafter, the number of re-interventions increased equally in both treatment groups, as reflected by a small but stable difference in favour of SES during follow-up. Similar patterns of a sharp increase in re-interventions within one year were observed in the STRATEGY and TYPHOON trials. However, in the present trial, the frequency of repeat target lesion revascularisations that were explicitly clinically driven was comparable between treatment groups after five years, despite the previously reported significant difference after one year12. Based on the results of this and other studies, the benefit of DES in STEMI patients regarding a reduced need for re-intervention seems to exist, but may be limited to the first year after stent implantation.
Safety of DES
Since the first concerns arose about the use of DES3-6, not one of the numerous studies that have been conducted as of this date have demonstrated inferior safety of DES. Nevertheless, the suspicion of a poorer clinical outcome at long-term follow-up after DES implantation for complex lesions persists, since there remain concerns that the results of leading meta-analyses do not reflect “real-world” clinical practice in which the large majority of DES is implanted for an off-label indication like AMI. However, large observational studies in AMI-patients did not report any differences in rates of death or re-MI either21,22. In addition, long-term mortality and re-MI rates were comparable between treatment groups in this and other randomised trials8-11. The incidence of re-MI in the current trial (SES 10.6% vs. BMS 13.7%) was slightly higher than reported in other trials (ranging from 5.2% to 8.9% in DES-groups and 4.3% to 13.3% in BMS-groups), which may be attributed to a stricter definition of re-MI.
Despite these reassuring safety outcomes, results of several meta-analyses comparing DES and BMS, including those referring to elective procedures, are contradictory regarding the risk of (late and very late) ST16,17,23,24. In addition to the inconsistency of analyses including patients with stable coronary lesions, there is evidence that DES implantation in patients with more complex lesions like in STEMI, is an independent predictor of ST25 and this association may be even more pronounced with those events occurring late7,26. Reports investigating the pathological mechanisms underlying ST in DES, like delayed arterial healing and limited endothelial coverage of stent struts, support these findings27,28. ST is a rare but serious event with significant morbidity and mortality, of which the incidence in some studies may be underestimated due to occurrence of “out of hospital” fatal events and variation in the applied definitions.
In the current trial, no increased risk of major adverse cardiac events was observed with either DES or BMS up to five years after the index procedure. However, it is remarkable that the benefit of SES, described after the first year12, is not sustained during long-term follow-up and a trend of more VLST was observed in SES-treated patients. This observation supports the previously expressed concerns, particularly if this trend will persist throughout subsequent years. Furthermore, VLST was significantly increased in patients with late stent malapposition versus those without late stent malapposition, which is observed more frequently after SES implantation29.
Despite all considerations regarding the risk of ST with DES, acomparison of outcomes between the different randomised trials is complex due to variation in design, sample size, definition of endpoints and stent polymer or drug. It is noteworthy though, that sample sizes of all the above mentioned randomised trials are underpowered to correctly detect differences in rare events like ST. Furthermore, a possible increased risk of (VL)ST after DES implantation should still be weighed against the presumed benefit of lower rates of restenosis and re-intervention.
Therefore, the very long-term outcomes of a large randomised controlled trial comparing DES with BMS in patients with acute myocardial infarction may contribute to a better insight into the efficacy and safety of coronary artery stenting with DES in STEMI patients.
Limitations
Several limitations of this trial should be noted for correct interpretation of the results. Firstly, outcomes cannot simply be translated into daily clinical practice. This study was a single-centre trial in aselected group of patients that followed a strict follow-up protocol. Moreover, the results only apply to SES in this specific study population. Secondly, the sample size calculations were performed for the initial purpose only12, and therefore the study was underpowered to detect differences in safety and efficacy endpoints.
Conclusions
The current trial showed no significant differences in death, re-MI and ST, nor in the need for recurrent revascularisation procedures in patients with STEMI that underwent primary PCI with implantation of SES compared to BMS after a follow-up period of five years. Although a trend was observed towards a reduction in re-intervention favouring SES, those procedures that were explicitly clinically driven were equally frequent in both the SES- and BMS-group. The observed tendency of more VLST in SES-treated patients is worrisome, since it may further compromise the long-term benefits of SES in STEMI patients.
Funding sources
This study was supported by The Netherlands Heart Foundation and by an unrestricted research grant from Boston Scientific, Inc., Nieuwegein, The Netherlands.
Conflict of interest statement
M. Schalij received research grants from Boston Scientific, Medtronic and Biotronik. The other authors have no conflicts of interest to declare.

