Abstract
Aims: Conflicting data exist about the safety of the sirolimus-eluting stent (SES) for patients with ST-elevation myocardial infarction (STEMI). Previous studies have reported delayed neointimal proliferation over SES with high incidence of adhering thrombus. This study was undertaken to assess the neointimal coverage and thrombus formation after SES implantation between patients with STEMI and those with stable angina pectoris (SAP).
Methods and results: We studied 23 patients with STEMI who underwent primary percutaneous coronary intervention (PCI) with SES and 18 patients with SAP who were treated with SES. Coronary angioscopic examination was performed 8.1±2.4 months after PCI. Neointimal coverage of the stent was classified into four grades (grade 0 to 3). Uncovered stent strut was defined as grade 0 or 1. All the patients with STEMI and 94% of patients with SAP had uncovered stent struts. There was no significant difference in minimum, maximum, and dominant neointimal coverage grade between STEMI and SAP. 96% of patients with STEMI and all the patients with SAP showed heterogeneous neointimal coverage. Thrombus adhering to uncovered stent struts was observed in eight patients after STEMI and in four patients after SAP (35% vs. 22%, p=0.38). There was no significant difference in the maximum colour grade of the plaques between STEMI and SAP (2.1±0.8 vs. 1.8±0.9, p=0.33). Most thrombus was observed at the site of yellow plaques (83%).
Conclusions: There was no significant difference in the neointimal coverage and thrombus formation between STEMI and SAP.
Introduction
Sirolimus-eluting stents (SES) reduce restenosis and repeat revascularisation procedures as compared with bare-metal stents.1,2 Widely publicised concerns arose recently about the incidence of late and very late thrombosis with the use of SES.3,4 Coronary angioscopic examination provides an opportunity to assess neointimal coverage over stent and adherent thrombus and plaque in the coronary artery. Previous studies have reported delayed neointimal proliferation over SES with high incidence of adhering thrombus, suggesting a relation to stent thrombosis.5 The sirolimus-eluting balloon-expandable stent in the treatment of patients with de novo native coronary-artery lesions (SIRIUS) led to the approval of the use of drug-eluting stent (DES) by the US Food and Drug Administration (FDA).1 However, SIRIUS excluded the patients with ST-elevation myocardial infarction (STEMI). Although some recent studies have suggested that SES could be safely and effectively used for patients with AMI without an increased risk of thrombosis, it is unclear whether there are any differences in stent neointimal coverage and thrombus formation after SES between patients with STEMI and those with stable angina pectoris (SAP).6,7
Using coronary angioscopy, we compared neointimal coverage and thrombus after SES implantation between patients with STEMI and those with SAP.
Methods
Study patients
This study consisted of 23 patients who underwent primary percutaneous coronary intervention (PCI) with SES for STEMI and 18 patients who underwent angioplasty with SES for SAP. Coronary angioscopy was performed 8.1±2.4 months after SES implantation. STEMI was diagnosed as chest pain consistent with ongoing myocardial ischaemia persisting >30 minutes with concomitant electrocardiographic ST elevation changes. SAP was defined as a positive stress test and no change in the frequency, duration, or intensity of symptoms within four weeks. All patients were receiving aspirin 100 mg/day and ticlopidine 200 mg/day from the index procedure to the time of follow-up examination. Neither glycoprotein II b/III a inhibitors nor clopidogrel was used because they have not been approved for clinical use in Japan.
Informed consent was obtained from each patient and this study protocol was reviewed and approved by the ethical committee of Hiroshima City Hospital.
Angioscopic examination procedure
Catheterisation was performed with a radial approach using 6 Fr sheath and catheters. After 5,000 units of heparin administration, selective coronary angiography was performed. Coronary angioscopic examination was performed with the angioscope VFS-1300 (Nihon Kohden, Tokyo, Japan) and optical fibre IF- 783V (Nihon Kohden). The outer section of the 4 Fr probing catheter (USCI) was used as the guide to insert the optical fibre into the coronary artery. The angioscopic observations were made while the blood was cleared away from the view by the injection of 3% dextran-40 through the probing catheter. The angioscopy images were recorded on a digital recorder.
Evaluation of the angioscopic findings
We assessed neointimal coverage over stent struts and the existence of red or white thrombus. We classified the degree of neointimal coverage over the stent struts in four grades (Figure 1). Grade 0 was defined as stent struts that were completely exposed (similar to immediately after the implantation), grade 1 when stent struts were visible with dull light reflexion, grade 2 when there was no light reflexion from the stent struts with slightly visible struts and grade 3 when stent struts were completely covered, and not seen through the neointima. Uncovered stent strut was defined as grade 0 or 1. Neointimal coverage was evaluated in the entire stented segments, and if different neointimal coverage grades of more than or equal to 1 grade were present, the neointimal coverage was judged as heterogeneous. Thrombus was defined on the basis of the criteria adopted by the European Working Group on Coronary Angioscopy. 8 The colour of the plaques under the stent was graded as 0 (white), 1 (light yellow), 2 (yellow), 3 (bright yellow), according to the sample colours presented in Figure 2.9 Because plaques of grade 0 were observed in at least one stent strut in all patients, the maximum colour grade of the plaques was assessed. Angioscopic evaluations were made by two angioscopic specialists blinded to the clinical status. This study was performed in a single centre.
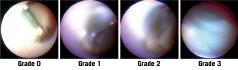
Figure 1. Angioscopic grading of neointimal stent strut coverage. Grade 0: Stent struts with complete exposure (similar to immediately after implantation). Grade 1: Transparent stent struts with dull light reflexion. Grade 2: Stent struts slightly visible, with no light reflexion from stent struts. Grade 3: Stent struts were completely covered, and not seen through neointima.
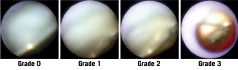
Figure 2. Grading of yellow plaque. The colour of the plaques was graded as 0 (white), 1 (light yellow), 2 (yellow), 3 (bright yellow).
Quantitative coronary angiography
Quantitative coronary angiography was performed using Centricity Cardiology CA1000 Cardiac Review 1.0 (Spa10) Operating System (GE Healthcare Ltd, Little Chalfont, Buckinghamshire, UK) before stenting, immediately after stenting, and also at follow-up with the same angle of projection (Table 1). Measurements were performed in end-diastole, preferably in two orthogonal projections or, if this could not be obtained, in the projection that best showed the diseased segment with as little foreshortening as possible. Reference diameter (millimetres), minimal lumen diameter (millimetres), and lumen diameter stenosis (percent) were measured after thrombus aspiration in patients with STEMI and before PCI in patients with SAP.
Statistical analysis
Data was shown as mean±SD. Differences between continuous variables were tested using the chi-square test. The Student’s t test was used for continuous variables. We considered anioscopic grades for neointimal coverage and plaque colour as continuous variables and performed the Student’s t test. We used the JMP statistical package version 5.0.1 J (SAS Institute, Cary, NC, USA) for all statistical tests. A significance level of 0.05 was used and 2-tailed tests were applied.
Results
Baseline characteristics
Baseline clinical characteristics of patients are shown in Table 2. Coronary angioscopy was performed 8.0±2.5 months after SES implantation for patients with STEMI and 8.3±2.4 months for patients with SAP. Hypertension and lesion location on the left ascending artery were more frequent in patients with STEMI than those with SAP. All the patients were on dual antiplatelet therapy at the time of angioscopic examination.
Angioscopic findings
Minimum, maximum, and dominant neointimal coverage grades are shown in Figure 3. All the patients with STEMI and 94% of patients with SAP had uncovered stent struts. There was no significant difference in minimum, maximum, and dominant neointimal coverage grade between STEMI and SAP. Heterogeneity of neointimal coverage grade is shown in Figure 4. Ninety-six percent (96%) of patients with STEMI and all the patients with SAP showed heterogeneous neointimal coverage. Thrombus was observed in eight patients after STEMI and in four patients after SAP (35% vs. 22%, p=0.38). In patients with STEMI, we observed red thrombus in six patients, white thrombus in one patient, and both thrombus in one patient. In patients with SAP, we found red thrombus in two patients, white thrombus in one patient, and both thrombus in one patient. All thrombus were observed as mural thrombus adhering to uncovered stent struts. None of them were detectable by angiography. Yellow plaques under stent struts were observed in 39 patients (95%). There was no significant difference in the maximum colour grade of the plaques between STEMI and SAP (2.1±0.8 vs. 1.8±0.9, p=0.33). Most thrombus was observed at the site of yellow plaques (83%).
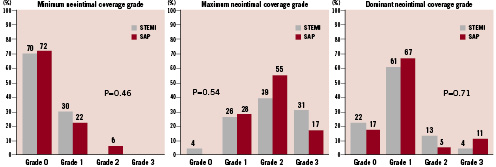
Figure 3. Minimum, maximum, and dominant neointimal coverage grades. 100% of STEMI had uncovered stent struts: 70% had Grade 0; 30% had Grade 1 as minimum neointimal coverage grade. 94% of SAP had uncovered stent struts: 72% had Grade 0; 22% had Grade 1 as minimum neointimal coverage grade. There was no significant difference in minimum neointimal coverage grade between STEMI and SAP. 83% of STEMI and 84% of SAP showed the dominant neointimal coverage grade of grade 1.
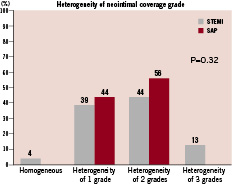
Figure 4. Heterogeneity of neointimal coverage grade. 96% of STEMI showed heterogeneous neointimal coverage: 39% showed the heterogeneity of 1 grade; 44% showed the heterogeneity of 2 grades; 13% showed the heterogeneity of 3 grades. 100% of SAP showed heterogeneous neointimal coverage: 44% showed the heterogeneity of 1 grade; 56% showed the heterogeneity of 2 grades. There was no significant difference in heterogeneity of neointimal coverage grade between STEMI and SAP.
Discussion
Although SES have dramatically reduced restenosis, previous studies have noted a possible increase in the risk of stent thrombosis.3,4 Daemen et al reported that late stent thrombosis occurred at a rate of 0.6 per 100 person-years of observation. The rate of stent thrombosis was five to six times higher when SES were implanted during AMI compared to SAP.10 However, recent studies demonstrated that SES could be safely and effectively used for patients with AMI without an increased risk of thrombosis.6,7 The Trial to Assess the Use of the Cypher Stent in Acute Myocardial Infarction Treated with Balloon Angioplasty (TYPHOON) was a multicentre randomised trail that compared the use of the CYPHER® stent (Cordis, Johnson & Johnson, Warren, NJ, USA) with any commercially available BMS during primary PCI of AMI.6 The use of the CYPHER® stent was associated with a lower incidence of target vessel revascularisation and similar incidences of death, MI and stent thrombosis as compared with BMS. The CYPHER® stent has received CE mark approval for the treatment of AMI. The Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial showed safety and efficacy of paclitaxel-eluting stents (PES) in AMI.11 However, because of limited data on long-term outcome, the safety of DES use for AMI still remains uncertain.
Coronary angioscopy allows direct visualisation of the surface colour and superficial morphology of plaque, thrombus, neointima, and stent struts. Previous studies using coronary angioscopic examination demonstrated that neointimal stent coverage after SES implantation was markedly delayed. Thrombus and yellow plaques were observed frequently after SES implantation as compared with the bare metal stents.5,12-14 However, patients with STEMI were excluded and direct comparisons of the angioscopic findings after SES implantation between STEMI and SAP were not performed. This is the first study that compares angioscopic findings after SES implantation between patients with STEMI and those with SAP.
Recently, Nakazawa et al reviewed 51 autopsy cases of patients who died after DES implantation and reported that vessel healing at the culprit site in AMI patients was delayed compared to those with SAP.15 However, since this was a pathological study, a selection bias is possible. Our study included 23 patients with STEMI and 18 patients with SAP after SES implantation. All patients were free from restenosis and thrombosis. We showed that there was no significant difference in angioscopic findings (neointimal coverage, thrombus, and plaque) after SES implantation between patients with STEMI and those with SAP. These findings suggest that the healing process after SES implantation is similar between STEMI and SAP.
The TYPHOON study showed that stent ARC definite and probable stent thrombosis occurred 4.8% in patients who received BMS and 4.4% in patients who received DES.16 Most stent thrombosis occurred in the first month, suggesting acute mechanisms due to the high content in thrombus. This current study confirms this hypothesis: after eight months, SES coverage is similar between patients with STEMI and SAP. Therefore, stent thrombosis after primary PCI for AMI is mostly related to acute thrombus and not to the type of stent implanted.
Dual antiplatelet therapy with clopidogrel and aspirin is the standard treatment in patients treated with DES for coronary artery disease for the prevention of stent thrombosis.17-19 Early discontinuation of dual antiplatelet therapy is a strong predictor of stent thrombosis.4,20-22 The current study showed that there was no significant difference in angioscopic findings after SES implantation between patients with STEMI and those with SAP and thrombus was observed adhering to uncovered stent struts in 1/4 to 1/3 of patients in patients with STEMI and SAP. Low-grade (grade 0 to 1) neointimal coverage and yellow plaque was associated with thrombus formation, which was similar to previous studies.5,11-13,23,24 Dual antiplatelet therapy may be needed to prevent stent thrombosis in the presence of low-grade neointimal coverage and yellow plaque after SES implantation. It is uncertain how long we should continue dual antiplatelet therapy to prevent stent thrombosis. Further studies are needed to assess how long dual antiplatelet therapy is needed.
Study limitations
First, our study is obviously limited by the small number of patients included. However, angioscopic studies are difficult to perform and require informed consent for angiographic follow-up. Angioscopic studies on a large cohort of patients is therefore difficult. Second, the dose of ticlopidine which we used was 200 mg which was approved dose in Japan. This was lower than the dose which was approved by the FDA. Third, we performed coronary angioscopic examination without an occlusion balloon, which is the difference with some other researchers. The difference in the type of angioscopic catheter might also have affected the results. However, most angioscopic findings are consistent between these two different devices. Fourth, the serial changes of thrombus and the colour of the yellow plaques was not assessed since coronary angioscopic examination was not performed during the base-line procedure. Fifth, we couldn’t assess neointimal function over stent struts by coronary angioscopy. Sixth, we didn’t perform IVUS and OCT in this study. The MISSION! Intervention study showed that late stent malapposition was observed more frequently in patients with SES for STEMI than those with BMS for STEMI.25-26 We though couldn’t assess stent malapposition by coronary angioscopy. Seventh, we assessed the angioscopic findings at eight months. A longer angioscopic follow-up is necessary to confirm the differences in the incidence of thrombus.
Conclusions
In this study, with angioscopic evaluation eight months after SES implantation during procédures for STEMI or SAP, neointimal coverage was heterogeneous. Thrombus was frequently observed adhering to uncovered stent struts and at the site of yellow plaques. There was no significant difference in neointimal coverage or thrombus between stents implanted in patients with STEMI or SAP.

