Abstract
Aims: Histopathology of DES restenosis remains unclear. The purpose is to examine restenotic tissue characteristics after sirolimus-eluting stent (SES) and comparing with that after bare-metal stent (BMS).
Methods and results: Intravascular ultrasound (IVUS), coronary angioscopy (CAS), and directional coronary atherectomy were performed simultaneously in 21 patients who presented restenosis after SES (n=13) and BMS (n=8). Mean time of restenosis was 10.8 months in the SES versus 7.5 months in the BMS. Typical “black hole’’, echolucent appearance by IVUS was observed in one SES case, and corresponded to a fibrin rich tissue by histology which appeared translucent tissue by CAS. CAS did not reveal red thrombus, but showed white thrombus in six SES versus two BMS (46.2% vs. 25.0%, p=0.597). Histology demonstrated various patterns after SES including thrombus, fibrin, inflammatory infiltrate, and collagen-matrix rich tissue, while thrombus component was not detected in BMS. Thrombus and fibrin deposition detected by either CAS or histopathology were observed more frequently in SES than in BMS group (92.3% vs. 25.0%, p=0.007).
Conclusions: Restenosis after SES and BMS have different clinical and histological patterns. SES restenosis may be frequently associated with thrombus component.
Introduction
Sirolimus-eluting stents (SES) significantly reduce the incidence of restenosis and the need for repeat revascularisation compared to bare-metal stents (BMS).1-8 Sirolimus is a potent cell cycle inhibitor which prevents smooth muscle cell proliferation. Necropsy reports have suggested that SES are associated with a greater degree of delayed healing compared to BMS9; however, these studies did not examine vessels with restenosis after SES implantation. While the time course and characteristics of neointimal hyperplasia after BMS implantation have been extensively investigated10,11 little is known about the restenotic process after SES implantation, which is infrequent but still occurs in 5-10% of patients. The aim of the present study was to investigate the clinical and histological characteristics of SES restenosis compared with BMS restenosis using intravascular ultrasound (IVUS), coronary angioscopy (CAS), and histopathology analysis of tissue obtained by directional coronary atherectomy (DCA).
Methods
Patient population
Consecutive patients presenting with SES and BMS restenosis underwent IVUS, CAS examination and DCA treatment, and a histological examination of tissue samples was conducted. Only patients who underwent PCI >6 months prior to developing restenosis were included. A total of 21 patients, in whom all planned procedures were performed successfully, were included in this analysis: 13 had been treated with SES (chest pain: n=) and eight received BMS. All patients provided written informed consent. The study was approved by the institutional ethics committee.
Procedure
After heparin (120 unit / kg) injection and 2.5 mg isosorbide dinitrate administration, IVUS was performed using a 40-MHz transducer with a 2.9 Fr imaging sheath (Atlantis Pro; Boston Scientific Corp., Natick, MA, USA). The ultrasound catheter was advanced >10 mm beyond the lesion, and an imaging run was performed to a point 10 mm proximal to the lesion with a motorised transducer pullback at 0.5 mm/s. Data were recorded onto a DVD for off-line analysis. After IVUS examination, the entire stented segment was observed with an angioscopic catheter (Vecmova Neo; Clinical Supply Corp., Gifu, Japan). The images and fluoroscopy were recorded on super VHS videotape for later analysis. The exact position of the angioscopic catheter at the observed segment was recorded on the angiogram to ensure a reliable comparison. During this procedure, an assistant monitored the light power to maintain a consistent degree of brightness on the target lesion observation. The colour of the observed neointimal surface was divided into two groups, white and yellow. Thrombus was defined by angioscopic findings of a red or white, superficial or protruding mass adhering to the vessel wall. After angioscopy, tissue was obtained from the stented lesions by DCA using Flexi-Cut (Abbott Vascular, Santa Clara, CA, USA). Procedure success rate was 100%, and no complications were observed. Tissue samples were immersion-fixed in 10% neutral-buffered formalin and processed for paraffin embedding in 4-μm sections. Sections were stained with haematoxylin and eosin stain (HE) as a routine stain, phosphotungstic acid-haematoxylin (PTAH) stain for fibrin, Alcian blue for proteoglycan antibodies against smooth muscle cell (SMC) specific α-actin, and antibodies for CD3 (T-cell lymphocyte) and for CD 68 (macrophage). Formations were defined as follows: poor: –, commonly observed: +, rich: ++. Quantitative coronary angiography (QCA) analysis was performed using the Cardiovascular Measurement System (CMS-MEDIS Medical Imaging System, Leiden, The Netherlands) at follow-up, following intracoronary administration of 2.5 mg isosorbide dinitrate. Quantitative IVUS measurement was performed using commercially available planimetry software (echoPlaque; INDEC Medical Systems, Santa Clara, CA, USA) by an independent experienced observer who was unaware of the clinical data.
Statistical analysis
Statistical analysis was performed using Stat View J-5.0 (SAS Institute, Inc., Cary, NC, USA). Frequency comparisons between the SES and BMS groups were performed with the χ2 test. Continuous variables were expressed as mean±SD and compared by unpaired Student’s t test. Statistical significance in all comparisons was p <0.05.
Results
Patient and lesion characteristics
Patient and lesion characteristics are summarised in Tables 1 and 2.
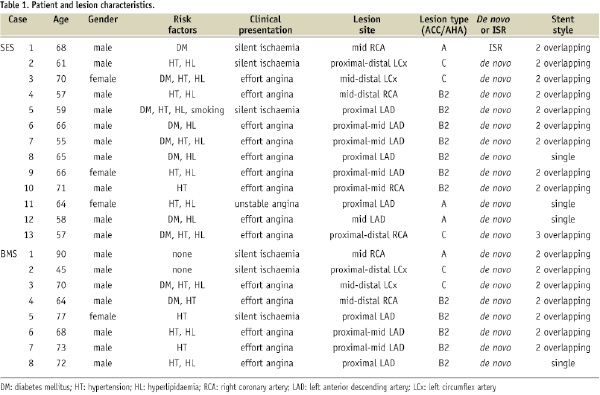
Except for one SES patient, all patients had taken aspirin as instructed. Antiplatelet (ticlopidine or clopidgrel) compliance was better in the SES group than in the BMS group (76.9 vs. 23.1%, p=0.070). The restenosis-free period after index percutaneous coronary intervention (PCI) was much longer in the SES group than in the BMS group (10.8±3.5 months vs. 7.5±3.1 months, p=0.042). In terms of clinical presentation of restenosis, four SES patients and two BMS patients presented with acute coronary syndrome. In one patient, an SES stent had been implanted in an in-stent restenosis site; the BMS group had no such cases. Ischaemia was present in all cases, including typical chest pain (n=6 in SES, n=3 in BMS) or positive scintigram or stress electrocardiogram test (n=7 in SES, n=5 in BMS) at more than six months after index PCI.
Angiographic, IVUS, and CAS findings
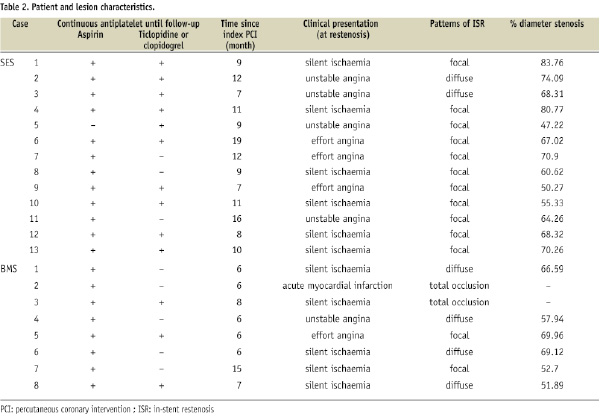
Angiographic, IVUS, and CAS findings are shown in Table 3.
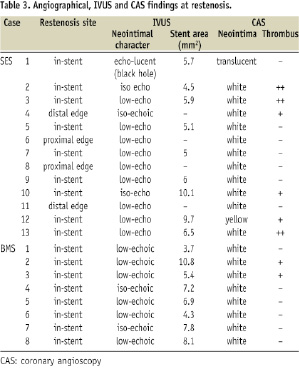
While the SES group included four cases of edge-restenosis with the remaining cases being in-stent restenosis, the entire BMS group presented with in-stent restenosis. There were no incidences of non-uniform distribution, fracture or stent malapposition. In both groups, nearly all cases presented low- or iso-echoic neointima excluding one “black hole” case in the SES group. There was no difference in the mean stent area at the restenosis site between the SES and BMS groups (6.5±2.0 mm2 vs. 6.8±2.3 mm2, p=0.782). While CAS revealed no red thrombus, white thrombus was found in six cases in the SES group and two cases in the BMS group (46.2% vs. 25.0%, p=0.597) (Figure 1).
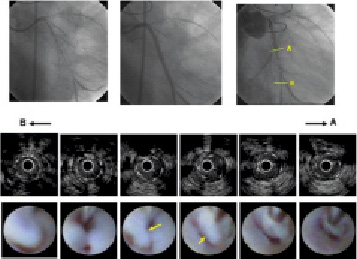
Figure 1. CAG, IVUS, and CAS findings of Case 2. Upper row: CAG images. Left panel: initial CAG. There is a diffuse long stenosis at left circumflex artery. Middle panel: post-SES (Cypher 3.0x28 mm; Cordis Corp., Miami Lakes, FL, USA) double implantation. Right panel: severe restenosis at SES implantation site after 12 months. Restenosis is visible in distal stent segment. Middle row: IVUS restenosis findings. IVUS indicates homogenous isoechoic lesions. Bottom row: CAS restenosis findings. White membrane with rich flapping white thrombus shown inside stent (yellow arrows).
The black hole case presented a white, thin translucent membrane (Figure 2A), a rare finding. In the SES group, only one case presented a yellow neointimal surface.
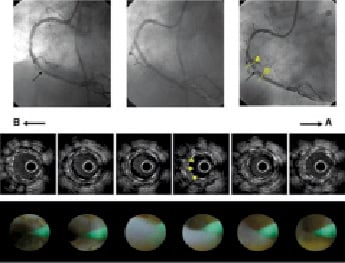
Figure 2. A. CAG, IVUS, and CAS findings of the black hole case. Upper row: CAG images. Left panel: initial CAG. There is stenosis at the mid-right coronary artery (black arrow). Middle panel: post-SES (3.0 x 13 mm) implantation. Right panel: severe restenosis in very short segment at SES implantation site after nine months (black arrow). Middle row: IVUS restenosis findings. The “black hole” is seen circumferentially (yellow arrows). Lower row: CAS restenosis findings. A thin, translucent membrane, a rare finding by CAS, is observed.
Histopathology
Neointimal histopathology findings are shown in Table 4.
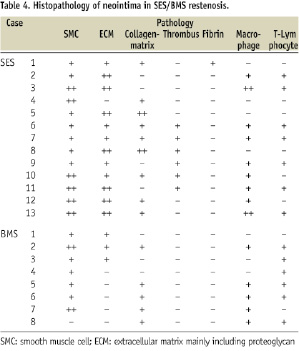
While the BMS group contained mainly SMC with a rich collagen matrix, the SES group contained a variety of components including thrombi, fibrins, rich inflammation cells, and collagen matrix (Figures 3A-C).
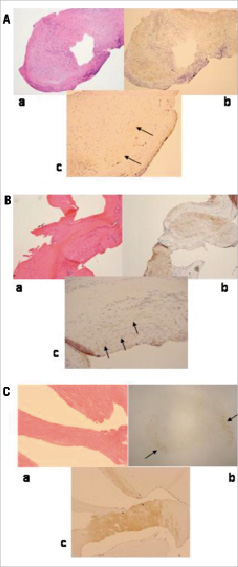
Figure 3. A. a) Rich inflammatory cells observed in HE. b) Moderate increase of SMC (brown area) with extra-cellular matrix (transparent area surrounding SMC area) observed. c) In terms of proteoglycans, very little decorin observed (black arrows). B. a) Rich collagen-matrix with slight cells observed in HE. b) Moderate increase of SMC (brown area) found. c) Moderate increase of extra-cellular matrix involving proteoglycans found (black arrows). C. a) Very rich collagen-matrix with slight cells was observed in HE. b) Few SMC (black arrows). c) Rich decorin with few versican observed (brown area).
Notably, thrombi were observed in six cases. The black hole case consisted of two components detected by HE staining: a rich collagen matrix area and a homogenous area. This collagen matrix area included some SMC, and the homogenous area was determined to be comprised of fibrins by PTAH staining. In terms of proteoglycans, very little versican or decorin were detected (Figure 2B).
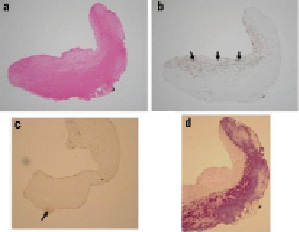
Figure 2. B. Case 1 histopathology results. a) Specimen consists of two components: homogenous tissue and collagen-matrix as seen in haematoxylin and eosin (HE) stain. b) α-SMA stain shows some smooth muscle cells in collagen-matrix (black arrows). c) There is little proteoglycans (versican and decorin) (black arrow). d) The violet area in the PTAH stain indicates rich fibrins corresponding to the homogenous tissue seen in HE.
Comprehensive interpretation of thrombus components
Thrombus components such as thrombus and fibrins, identified by CAS or histopathology, were observed more frequently in the SES group (cases 2,3,4,6,7,10,11,12,13; n=12/13) than in the BMS group (cases 2,3; n=2/8) group (92.3% vs. 25.0%, p=0.007).
Discussion
Aside from animal models and autopsy data of patients who died after SES implantation, very little examination of neointimal characteristics after SES implantation has been done.2,11,12,13,14 IVUS and CAS have limited ability to define the characteristics of in vivo neointima: IVUS cannot detect the precise characteristics of neointima, and CAS makes it possible to see only the surface of neointima. Furthermore, tissue samples collected through DCA are too small to allow identification of all neointimal characteristics. Thus, we thought that a more comprehensive examination, using various modalities, was very important to define the characteristics of neointima. This is the first clinical study to simultaneously assess post-SES implantation neointima using IVUS, CAS, and DCA histopathology. This examination could perhaps begin to answer questions regarding different times before restenosis presentation, or the occurrence of late thrombosis.
Clinical presentation of post-SES restenosis, including the possibility of occult thrombosis
In this study, four of 13 patients had symptoms of unstable angina >7 months after SES implantation. Because these four cases showed neither ST-elevation on electrocardiogram nor total occlusion under CAG, they were not considered to be clinical late thrombosis. Though no thrombus components could be obtained by DCA, probably because of the limited sample volume of DCA, CAS did identify two cases of white flapping thrombus, and histopathology identified the existence of rich fibrin in one case. Although these cases are not the typical late stent thrombosis (LST) described in previous reports15,16, they could be occult thrombosis. Because this study revealed that post-SES restenotic lesions commonly include a thrombus component, this may be an example of the thromborestenosis phenomenon.
IVUS and CAS images
In recent studies, IVUS examination of vasculature several months post SES implantation suggested that stent under-expansion or non-uniform distribution may be linked with SES restenosis17-19, however, no neointimal characteristics were observed. Though the black hole case in this study, an echolucent area within a stent strut, was similar to the finding observed in cases of failed brachytherapy and SES implantation in saphenous vein grafts20,21, it was not a common finding. Thus, IVUS identification of SES restenotic lesions remains ambiguous. The average minimum stent area at SES restenosis site in this study was over 5 mm2, as recommended by a previous report18, and all cases in this study were found to have optimal apposition. Thus unfortunately, compared to BMS restenosis, there were no specific IVUS discoveries about SES restenosis except for the black hole case. As a result, defining neointimal characteristics post SES implantation by IVUS alone may prove difficult. While CAS analyses after SES implantation reveal the presence of thrombus at non-restenosis sites, there are no reports indicating the presence of neointimal tissue at restenosis sites22,23. This study showed a variety of CAS findings at post-SES implantation restenosis sites. While previous studies report frequent observation of thrombus above the yellow plaque which remained at uncovered stent segments in SES cases, our study saw the thrombus above, not only the uncovered stent segments, but also the covered stent segments at the restenosis sites. Until now, it has been thought that because sirolimus directly inhibits SMC migration and proliferation, thrombus disappearance might be delayed due to neointimal growth suppression.22 However, this hypothesis fails to explain those cases in the current study in which neointimal hyperplasia proliferation was found at restenotic lesions. The existence of thrombus above post-SES neointima may suggest that post-SES neointima lacks anti-thrombotic ability. While neointimal coverage after BMS implantation makes it possible to prevent thrombosis, neointimal hyperplasia covering SES struts seems to lack that ability.
Histological findings
Human coronary histopathology after BMS implantation has been well documented.10,11 At >6 months post BMS implantation, the major component is SMC with collagen matrix, and chronic inflammation remains only near struts. Thrombus, including fibrin deposits, does not exist. In this study, each SES case takes exception to this BMS theory. Some cases presented rich inflammatory cells, thrombus, or fibrins at follow-up. Prolonged arterial healing after SES implantation may simply be a difference in degree, or perhaps post-SES neointimal hyperplasia mechanisms differ from those occurring after BMS implantation. Our results clearly show that the degree of healing after SES implantation is quite different from that of BMS. A recent histological examination of drug-eluting stent restenosis found fibrinoid, which represented an incomplete healing response.12 Our data supports the results of that report and confirms the link between the restenosis-related thrombus components and the thrombus appearance detected by CAS. Histological assessment of black hole tissue extracted from human coronary arteries after brachytherapy determined that it is composed of a hypocellular matrix with areas of abundant proteoglycan24, however post-SES implantation black hole tissue has yet to be identified. In this study, we found that rich fibrin existed in the area corresponding to the black hole. Though this finding was different from that of brachytherapy, the hypocellularity of intraluminal tissue, such as proteoglycans or fibrin, may explain the echolucency found on ultrasound.
Study limitation
This is a small study, due to the difficulty in complex procedures and low incidence of SES restenosis. A larger study would be needed to expand the results of this study.
Conclusion
This is the first study to simultaneously analyse, using IVUS, CAS, and histopathology, neointimal characteristics in patients who presented with SES restenosis on visual examination. After several months, a high rate of thrombus components remained, but without apparent late thrombosis presentation. It seems certain that the mechanism of neointimal formation after SES implantation is different from that of BMS. Taking the results of this study into consideration could be important for follow-up of patients treated with SES.
Acknowledgements
The authors thank Eiji Noguchi, Erika Miura, and Yoshie Takanashi for their technical assistance.

