Abstract
Aims: Drug eluting stents (DES) have been used routinely in a wide variety of clinical situations. The impact of DES on reducing restenosis has not been uniform across complex subsets and limited data is available examining predictors of restenosis in unselected population.
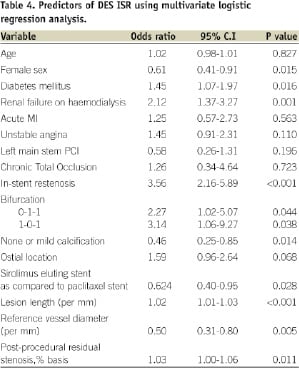
Methods and results: We investigated predictors of angiographic restenosis in an unselected population. The study population consisted of 4,143 lesions and angiographic follow-up was available for 3,020 (73%) lesions in 1,885 patients. The intravascular ultrasound (IVUS) was used in 95% of the patients during the procedure. Angiographic restenosis was seen in 339 (11.2%) lesions and target lesion revascularisation was performed in 290 (9.6%) lesions. The patient population included large numbers of renal failure patients on haemodialysis, ISR, and Type C lesions with routine use of intravascular ultrasound. We identified diabetes mellitus, renal failure, Type C lesions, calcified lesion, tortuous lesion, ISR, long lesion, small baseline diameter and final vessel diameter as predictors of restenosis. On multivariate analysis diabetes (OR 1.45, 95% CI 1.07-1.97, p= 0.01), renal failure on haemodialysis (OR 2.02, 95% CI 1.37-3.27, p=0.001), ISR (OR 3.56, 95% CI 2.16-5.89, P<0.001), lesion length (OR 1.02, CI 1.01-1.03.P<0.001), reference vessel diameter (OR 0.50, 95% CI 0.31-0.80, p=0.005) and post-intervention IVUS lumen area (p<0.001) were independent predictors of angiographic restenosis. Female gender (OR 0.61, 95% CI 0.410.91, p=0.015) was found to have a negative correlation for ISR. We did not find any significant difference in restenosis between the usage of sirolimus and paclitaxel eluting stents.
Conclusions: DES usage was associated with overall low in-stent restenosis and we have identified several clinical, angiographic, and IVUS predictors of angiographic restenosis in unselected patients with complex anatomy.
Introduction
Drug eluting stents (DES) have been shown to reduce the incidence of restenosis as compared to bare metal stents in several randomised trials1-4. Moreover, the utilisation of DES has exploded, resulting in it being considered as a default strategy and it is being used for a wide variety of clinical and off-label indications. There has been an increasing complexity of coronary lesions like patients, with in-stent restenosis, haemodialysis, multivessel disease, etc., being offered treatment with percutaneous revascularisation using DES. Therefore, even in the era of DES, treatment of such complex lesions and patients subsets is associated with a higher rate of ISR. Some studies have shown lesion length, minimal lumen diameter, stent type, female gender, diabetes, ostial location, and ISR as some of the predictors of DES ISR clinically or angiographically5-9. These studies have been somewhat contradictory in predicting various potential factors related to DES ISR.
We therefore analysed the predictors of in-stent restenosis following implantation of DES in an unselected population of real world patients.
Methods
Patient population
All consecutive patients undergoing treatment with one or more DES between May 2004 and December 2007 at our hospital (Toyohashi Heart Centre, Japan) were included in the study population. During this period 4,143 lesions in 2,586 patients were treated with drug eluting stents and angiographic follow-up was completed at nine months following stent implantation in 3,020 lesions (73%) in 1,885 patients. These 3,020 patient lesions were analysed to correlate the predictors of DES ISR.
Procedural details
Patients received either sirolimus eluting stents (Cypher, Johnson & Johnson Cordis Corp; Miami Lake, FL, USA) or paclitaxel eluting stents (Taxus, Boston Scientific, Natwick, MA, USA). The procedure was performed at operator’s discretion using standard practice at that time, and intravascular ultrasound usage was routine throughout the procedure. All patients were pre-treated with aspirin (100 mg) and ticlopidine (200 mg), or clopidogrel (300 mg). Long term aspirin (100 mg) and ticlopidine (100 mg) was continued after the procedure. During the procedure, weight adjusted heparin was administered to achieve the ACT > 300 seconds, and none of the patients received glycoprotein inhibitors. All patients were scheduled for follow-up angiography at nine months, or before if indicated clinically.
Definitions
Angiographic success was defined as < 30% residual stenosis with thrombolysis in myocardial infarction grade 3 flow.
Bifurcation lesions were classified as per the Medina classification10 and included the lesions involving the main or side branch.
Tortuosity was defined as the angulation at the lesion site and classified as mild (< 45 degrees), moderate (45-90 degrees), and severe (> 90 degrees). Calcification was defined on the basis of both angiography and IVUS.
All demographic, clinical, and procedural data were collected and entered into the dedicated database.
Coronary angiograms during procedure and at follow-up were analysed by two experienced observers after administration of intracoronary nitroglycerine administration using quantitative angiographic analysis system (CMS-MEDIS, medical Imaging Systems, Leiden, The Netherlands). Lesion length, reference diameter, minimal lumen diameter (MLD), and % diameter stenosis were measured. Intravascular ultrasound (IVUS) was performed with available equipment and minimal lumen area pre and post stenting were measured along with qualitative analysis. ISR was defined as the diameter stenosis more than 50% in two perpendicular views occurring inside stent, or 5 mm on either side of the stent at follow-up angiography.
Statistical analysis
Data for continuous variables were expressed as means±SD and the categorical variables were expressed as frequencies. Continuous variables were compared using Student’s t test and categorical variables using chi-square test or Fischer’s exact test. A univariate and multivariate predictor analysis using logistic regression binary angiographic in-stent restenosis was performed. The predictors were chosen by step-wise procedure criteria using an entry criterion of 0.05 with a removal of 0.10 in the logistic regression model. The Hosmer-Lemeshow goodness-of-fit statistics showed that the model adequately fits the data (Chi-square= 5.750, df=8, Sig=0.675). The odds ratio for continuous variables reflects changes in per unit of the measurement. All tests were two tailed and p<0.05 was considered significant.
All statistical analyses were performed using SPSS Version 15.0 statistical software (SPSS Inc., Chicago, IL, USA).
Results
A total of 3,020 lesions were seen in 1,885 patients who completed angiographic follow-up, thus constituting the study population of the present analysis which is based on this group of patients. The mean time to follow-up angiography was seven months, ranging between six and ten months. The groups with and without follow-up angiography did not differ significantly with regard to clinical, lesion, or procedural characteristics. However, the 701 patients without follow-up angiography were older (p<0.01), and had higher incidence of previous CABG (P<0.01) than the 1,885 patients in whom follow-up angiography was performed.
ISR was angiographically documented in 339 lesions (11.2%) and target lesion revascularisation was performed in 290 lesions (9.6%).
Baseline demographic and procedural characteristics are shown in Tables 1 and 2.
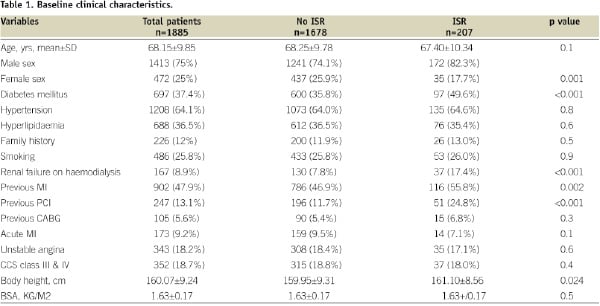
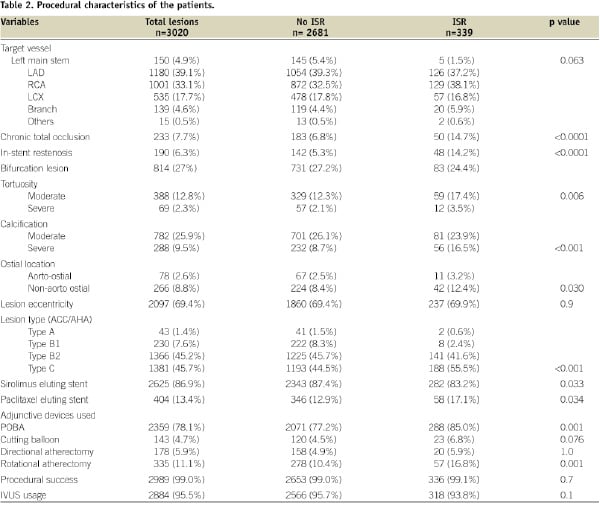
Patients with DES ISR are more likely to be diabetic and were more likely to have previous myocardial infarctions. There were significantly more patients with renal failure on haemodialysis presenting as restenosis. Other variables were similar in both groups.
On comparing procedural and angiographic variables as shown in Tables 2 and 3, there were more patients with chronic total occlusion, ISR, tortuous lesions and with severe calcification in DES ISR group.
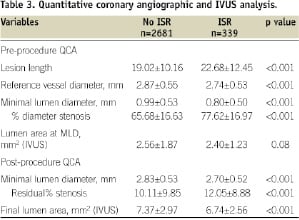
There were more patients with lesions at ostial location and ACC/AHA Type C lesion with ISR. There were significantly more patients with ISR following the usage of paclitaxel eluting stents.
On angiographic evaluation long lesion, small reference vessel diameter and residual percent stenosis and small final lumen area were significant factors in patients with DES ISR.
Predictors of in-stent restenosis
There were several factors identified on univariate analysis such as diabetes mellitus, renal failure on haemodialysis, previous myocardial infarction, chronic total occlusion, ISR, tortuous and calcified lesions and Type C lesions which are shown in Tables 1 and 2. Long lesion, small vessel, residual stenosis and final lumen area were also predictive of DES ISR as shown in Table 3. There was more restenosis seen in lesions treated with paclitaxel eluting stents (14.3% vs. 10.1%; p=0.034).
On multivariate analysis, as shown in Table 4, diabetes mellitus and patients with renal failure on haemodialysis (OR 2.02, CI 1.37-3.27, P=0.001), ISR lesions (OR 3.56, CI 2.16-5.89, p<0.001), bifurcation lesions and lesions with severe calcification were predictors of DES ISR. Lesion length (OR 1.02, CI 1.01-1.03, p<0.001), reference vessel diameter (OR 0.50, CI 0.31-0.80, p=.005) and final residual stenosis (OR 1.03, CI 1.00-1.06, p=0.011) were also shown to be significant predictors of DES ISR. The female sex, and the usage of sirolimus eluting stents as compared to paclitaxel eluting stents, have shown protective effect on restenosis.
Intravascular ultrasound was performed in 95% of the patients before and after stent implantation. We have identified pre-procedure lumen area (2.56±1.87 mm2 vs. 2.40±1.23 mm2, p=0.82; S.E of diff=0.09, 95% CI -0.02-0.34) at the MLD site as the predictor of restenosis. In our study, the final post-intervention lumen area (7.37±2.97 mm2 vs. 6.74±2.56 mm2, p<0.001, SE of diff= 0.17, 95% CI 0.28-0.96) was also found to be significant predictor of restenosis on univariate analysis.
Discussion
In our series of patients we have identified several baseline and angiographic predictors of DES ISR. Patients with renal failure on haemodialysis, ISR lesions and diabetes mellitus still remain at high risk of ISR in DES era. Lesion length, vessel diameter and residual final stenosis are also significant predictors of DES ISR as were seen in BMS ISR. The overall incidence of ISR remains low in these complex patient subsets with high frequency of Type B2/C lesions, renal failure patients and patients with ISR.
Drug eluting stents have shown to significantly reduce the incidence of angiographic restenosis and target vessel revascularisation as shown in several randomised trials1-4. Reference vessel diameter has been regarded as significant predictor of restenosis in randomised trials. In the SIRIUS trial11, the rates of angiographic restenosis were 17.6% in small vessels and 1.9% in large vessels. Similarly in the TAXUS V trial12, there were high restenosis rates in small vessels (31.2% vs. 3.5%). Our study also showed reference vessel diameter as an independent predictor of restenosis.
Post-intervention residual MLD has shown to be important predictor of restenosis in the bare metal stent era and stent under-expansion still remains the important cause of DES ISR. Fuji et al13 has shown significant correlation between minimal stent area on IVUS examination and DES ISR in patients undergoing sirolimus implantation for the treatment of ISR. Our study also shows the final residual diameter stenosis as an independent factor of restenosis.
Kastrati et al8 have identified predictors of restenosis in a consecutive series of patients, and reported vessel size, final diameter stenosis and drug eluting stent type as the strongest predictors. The overall angiographic restenosis was 13.0% which is similar to our series. Lee et al7 reported an overall angiographic restenosis rate of 8.8% and identified the use of paclitaxel eluting stent, post-intervention MLD and lesion length as independent predictors of restenosis following DES usage. Similarly, Roy et al9 have identified, age, hypertension, unstable angina, left anterior descending artery intervention, lesion length, and lack of IVUS guidance as predictors of ISR requiring revascularisation following DES usage.
Diabetes has been considered as the predictor of restenosis following coronary angioplasty and with bare metal stent usage14,15. However, data from recent studies with the application of DES has shown conflicting results. SIRIUS trial substudy16 and Lemos et al5 have shown diabetes as a predictor of restenosis. Kuchulukanti et al17 have shown higher MACE rates at six months, including target vessel revascularisation (6.0% vs. 2.7%, p=0.01).
However, some studies have not shown diabetes as a correlate for ISR following DES usage7-9,18-20. In our study, we have found diabetes mellitus as an independent predictor of angiographic restenosis.
Studies have shown similar rates of angiographic restenosis and one year MACE rates between men and women with the usage of sirolimus eluting stents in post hoc analysis26. This study showed similar binary restenosis rates (6.4% vs. 6.3%), despite worse baseline clinical profile, including old age and diabetes in females. In our study, the female sex was shown to have negative correlation with restenosis following SES usage. This could be due to the variations in the treatment and other unknown factors. This needs to be confirmed in other studies.
Our study included large number of patients with endstage renal failure on haemodialysis, and the outcomes in this high risk group is still suboptimal. The restenosis rates following coronary angioplasty and stenting is high in patients on haemodialysis. Several small studies have reported the restenosis rates ranging from 20% to 50% in this subgroup, with not much differences seen between bare metal and drug eluting stents21-25. Our study included 9% of our patients on haemodialysis; angiographic restenosis rates were 22% and this was one of the major independent predictors of restenosis. These results are in line with the published literature. Patients on haemodialysis are at particular risk of restenosis as they may have a relatively high rate of diabetes and frequently present with complex coronary artery disease such as long, calcified lesions.
Studies in the bare metal stent era have shown the benefit of IVUS guidance in percutaneous coronary intervention. In the Can Routine Ultrasound Influence Stent Expansion (CRUISE)27 and TULIP28 studies, IVUS guided percutaneous coronary intervention compared with angiographic guidance alone was associated with significantly better angiographic and clinical outcomes. There is no similar data available utilising DES. In our study, the IVUS was performed routinely and we have identified final lumen area as significant predictor of restenosis.
Routine IVUS guidance can potentially reduce the incidence of restenosis in the DES era by allowing complete expansion of the stent and complete coverage of the lesion. This needs to be tested in a randomised study.
Conclusions
Our study shows low angiographic and target vessel revascularisation rates with the usage of DES in an unselected, real world population. Our study population included a high percentage of patients with haemodialysis, ISR, and Type C Patients showing overall low occurrence of restenosis. This could be potentially be associated with very high frequency of IVUS usage. We have identified several predictors of restenosis including IVUS measured preprocedure minimal lumen area as well as post-stenting final lumen area.
Limitations
Our study has some potential limitations. Firstly, this is a retrospective analysis from a single centre, but it includes all comers with no exclusions. Secondly, the choice of DES and procedural technique was at the operator’s discretion and, thirdly, incomplete angiographic follow-up can lead to some potential error.

