Abstract
Aims: We aimed to assess the influence of different sirolimus analogues released from a uniform stent platform on re-endothelialisation and vascular healing responses.
Methods and results: Bare metal stents (BMS) were coated with a fluoropolymer containing everolimus (EES), sirolimus (SES) or zotarolimus (ZES) to generate drug-eluting stents (DES) with identical stent backbones, drug loads and release kinetics. DES constructs and control BMS were implanted into the iliac arteries of rabbits and were analysed at 14 days by scanning electron microscopy (SEM) and confocal microscopy for en face evaluation of endothelialisation (n=6 for each stent), or at 28 days to determine histomorphometric characteristics (n=11 for each stent). SEM analysis revealed low degrees of strut re-endothelialisation within the DES without differences among groups, while the BMS control showed almost complete endothelialisation. Percent stenosis was significantly reduced in all DES compared to BMS. Strut-based fibrin analysis revealed significantly greater deposition in the DES compared to BMS, with EES showing maximum fibrin deposition among the DES groups.
Conclusions: Sirolimus and its derivatives have similar effects on endothelial regrowth and neointimal thickening. The observation of greatest fibrin deposition in the experimental EES group indicates that everolimus may affect vascular healing differently.
Introduction
Recent scientific efforts in interventional cardiology have focused on the elucidation of the principles underlying delayed arterial healing following drug-eluting stent (DES) implantation1. Despite substantial advances in antithrombotic therapy for patients receiving DES, the deficient re-endothelialisation of DES is recognised as an important risk factor for the occurrence of late stent thrombosis. Apart from incomplete and dysfunctional re-endothelialisation, human autopsy studies have revealed hypersensitivity reaction, malapposition and uncovered stent struts (lacking neointimal coverage) as important additional risk factors2,3.
Bearing this in mind, developments in DES technology have focused on the design and biocompatibility of stent coatings as important variables which need attention. While polymers are known to trigger increased inflammatory reactions4,5, the specific impact of the antiproliferative drugs used has not been investigated. Previously, we reported that differences in vascular response and the time course of re-endothelialisation amongst commercially available DES are relevant, with the newer-generation DES showing more favourable healing properties6. However, the precise contribution of individual stent components –backbone, polymer and antiproliferative substance– could not be distinguished.
In the current study, we sought to compare the biological effects of currently used derivatives of sirolimus on vascular healing. In particular, we aimed to study the differential impairment of quantitative and qualitative re-endothelialisation and vascular healing characteristics utilising dedicated stents coated with different limus drugs.
Materials and methods
DEVELOPMENT OF EXPERIMENTAL DES CONSTRUCTS
The composition and development of the experimentally designed limus DES were based on the fabrication technique utilised in the XIENCE V® stent system (Abbott Vascular, Santa Clara, CA, USA). Vinylidene fluoride and hexafluoropropylene copolymer served as the drug matrix layer for each of the three limus drugs: everolimus, sirolimus and zotarolimus. The acquired carrier/drug solutions were coated onto the ML VISION™ AS™ F-90 cobalt-chromium stent system (3.0 mm×12 mm; Abbott Vascular, Santa Clara, CA, USA) at a total drug load of 40 µg and a polymer thickness of ~4.6 µm. All DES constructs showed 60% elution of the drug within 30 days as assessed by in vitro release kinetics. The following stent constructs were obtained: everolimus-eluting stent (EES), sirolimus-eluting stent (SES) and zotarolimus-eluting stent (ZES).
ANIMAL STUDY DESIGN
The study protocol was approved by the responsible authority (Regierung von Oberbayern, AZ 55.2-1-54-2531-109-07) implementing the German Animal Welfare Act. Animal housing and care were in agreement with the Directive 2010/63/EU of the European Parliament, compliant with the US National Institutes of Health Guide for the Care and Use of Laboratory Animals.
ANIMAL MODEL AND STUDY ENDPOINTS
A total of 34 healthy male New Zealand White rabbits (Charles River, Sulzfeld, Germany), weighing 3.5-3.8 kg, were included in this study. Animals were randomised to four different treatment groups consisting of the three limus DES constructs and the bare metal stent backbone as control (BMS control). Twelve animals were assigned for the assessment of re-endothelialisation at 14 days (n=6 stents for each group: EES, SES, ZES and BMS control), while 22 animals were assigned for histopathologic analysis by light microscopy at 28 days (n=11 stents for each group: EES, SES, ZES and BMS control).
STENT PLACEMENT AND TISSUE PROCESSING
For surgical procedures animals were anaesthetised in a controlled manner with propofol (Fresenius Kabi, Bad Homburg, Germany) and repetitive boli of 0.01 mg/kg fentanyl IV (Fentanyl; DeltaSelect, Dreieich, Germany). Preemptive analgesia was secured by administration of 0.025 mg/kg buprenorphin (Temgesic®; Essex Chemie AG, Luzern, Switzerland) s.c. pre- and post-surgery at eight-hour intervals for up to two days post-surgery. Throughout the surgery procedure animals were intubated, mechanically ventilated and monitored for vital signs (pulse oximetry and capnography). To achieve appropriate anticoagulation, 40 mg aspirin (Aspirin IV; BayerVital, Leverkusen, Germany) and 150 IU/kg heparin (Liquemin; Hoffmann-La Roche AG, Grenzach-Wyhlen, Germany) were administered IV at the time of catheterisation, continued on a daily oral dose of 40 mg aspirin (Aspirin Migräne; Bayer Vital, Leverkusen, Germany) until the time of study termination. Arterial access was obtained by deploying an arterial sheath in the common carotid artery. Following arterial denudation, utilising a standard angioplasty balloon catheter (VOYAGER RX, 3.0 mm×8.0 mm; Abbott Vascular, Santa Clara, CA, USA), stents were deployed at nominal pressure (9 atm) in each external iliac artery. Post-procedural angiography was performed to verify vessel patency. At study termination, animals were anaesthetised in the same manner to conduct a follow-up angiogram followed by euthanasia with pentobarbital overdose IV in the still anaesthetised animal. Subsequently, arteries were flushed with 500 ml of heparinised ringer lactate to remove blood. Stented arteries were fixed in situ with 10% neutral buffered formalin before harvest and kept in 4% neutral buffered formalin until further histological preparation.
DATA ANALYSIS
ASSESSMENT OF ENDOTHELIAL SURFACE COVERAGE BY SEM AND IMMUNOSTAINING
Formalin-fixed arterial segments of the 14-day animal group were cut longitudinally, opened and subjected to en face SEM and en face immunostaining for CD31/PECAM-1, as previously described6.
For SEM evaluation, serial images were recorded at x10 magnification using a Hitachi Model 3600N scanning electron microscope and digitally assembled to provide an overview of the entire luminal stent surface. Higher magnification (x200) allowed for visualising the strut surface with regard to composition of the adhering cells. Endothelial cells, inflammatory cells and platelets were reliably identified by their characteristic morphology6. Endothelial surface coverage was assessed above and between struts by measuring the respective areas of endothelialisation relative to the total strut area and the area between struts (IPLab™ for Mac OS X; Scanalytics, Rockville, MD, USA), respectively, and expressed as percent endothelial cell coverage above and between struts.
Sequential images of the immunostained specimen were taken at x100 magnification utilising confocal microscopy (Zeiss Pascal, Jena, Germany). The extent of endothelial cell coverage was based on a positive CD31/PECAM-1 signal and was visually semi-quantified as a percentage of the total in-stent vessel area above and between struts. Adjacent non-stented segments served as positive controls for immunostaining.
HISTOPATHOLOGICAL ASSESSMENT BY LIGHT MICROSCOPY
Twenty-eight days after implantation, the formalin-fixed stented vessel segments were dehydrated and embedded in methyl methacrylate polymerisation mixture. Each specimen was divided into its proximal, mid and distal segment. Sections were obtained from each segment (three sections per stent) and stained by haematoxylin and eosin (H&E), Movat’s pentachrome and Carstairs stains. Computerised planimetry (Cell^F Software; Olympus, Hamburg, Germany) was performed on all stented sections as previously described7. Vascular injury, fibrin deposition and inflammatory responses were evaluated in accordance with established scoring systems8,9. Additionally, in sections stained for fibrin (Carstairs stain), the area within the neointima containing fibrin was digitally measured and expressed as a percentage of the neointimal area positive for fibrin. Endothelialisation was assessed as a percentage of endothelium occupying the luminal circumference of the artery.
STATISTICS
Data was assessed for normal distribution by Shapiro Wilks W test. ANOVA or Wilcoxon rank sum test were used to calculate the significance of differences between DES groups and BMS control according to the statistical distribution of data. Each pair Student’s-t or all pairs Tukey HSD tests were used to compare the data among the DES treatment groups where appropriate. A value of p<0.05 was considered statistically significant.
Results
Thirty-six rabbits were included in the study. Two rabbits were euthanised shortly after stent implantation due to arterial dissection. All other stents were widely patent at the time of euthanasia without evidence of migration or aneurysm formation.
ENDOTHELIAL SURFACE COVERAGE ASSESSED BY SEM
At 14 days after stent implantation the luminal surface of the DES constructs showed a similar tissue coverage profile at low power magnification (x10), with the majority of the struts being uncovered (Table 1). Middle DES segments in particular showed impaired endothelialisation (Figure 1). Endothelial cells identified at high power (x200) magnification predominantly showed an elongated morphology in the DES constructs (Figure 2). Bare metal control stents showed significantly greater endothelial coverage above struts (Table 1) with endothelial cells exhibiting cobblestone morphology (Figure 2). Overall there was significantly less re-endothelialisation above stent struts in the DES groups as compared to BMS control, while there was no statistical difference among the different DES groups (Table 1). All treatment groups were found to have a similar high degree of endothelial regrowth in the areas between struts (Table 1).
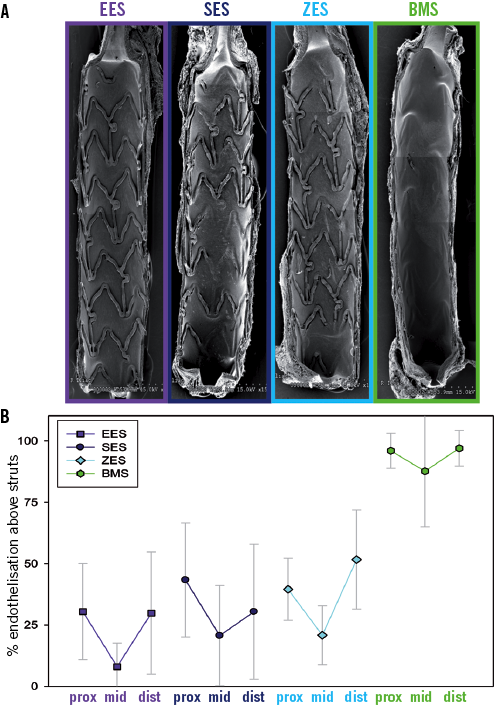
Figure 1. Quantitative distribution of endothelialised strut area among DES constructs and BMS control at 14 days post-stenting. A) Low-power view of the entire luminal surface of each stent visualised per SEM. While the BMS control shows almost complete tissue coverage of the strut surface, the DES constructs show uncovered and clearly visible stent struts, with only the very proximal and distal regions being buried under a tissue layer. B) The distribution of relative endothelialisation above struts along the longitudinal axis (proximal [prox], middle [mid] and distal [dist] segments) is shown as a line graph for each stent. The data mirrors the observation described above.
IMMUNOSTAINING FOR CD31/PECAM-1 ENDOTHELIAL CELL MARKER
Assessment of endothelialisation by CD31/PECAM-1 staining using confocal microscopy demonstrated weak staining (<30% of the visualised surface) between and above struts with no observable differences among the DES constructs at 14 days (Table 1). CD31/PECAM-1 expression over the struts was limited to focal areas within the proximal and distal stented regions. By contrast, the BMS control group showed greater CD31/PECAM-1 expression (Figure 2), which was more evenly distributed as compared to the DES groups (Table 1).
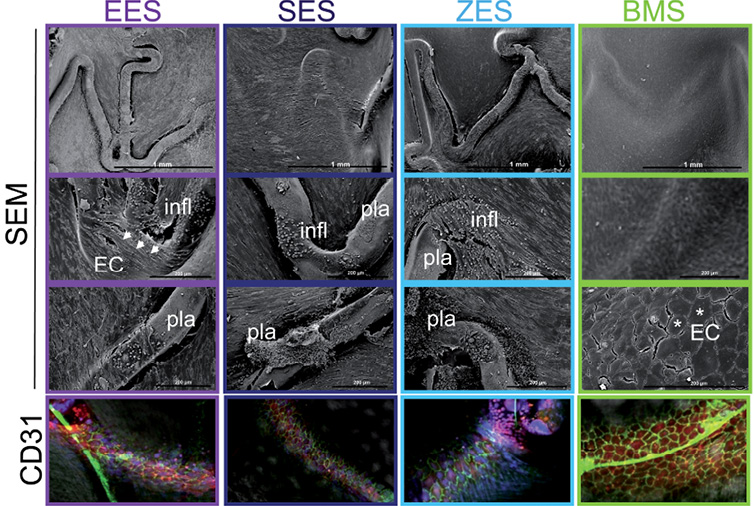
Figure 2. Microphotographs of en face luminal stent surfaces analysed per SEM and confocal microscopy. Higher magnification of SEM pictures within the stented region of DES shows elongated, spindle-shaped endothelial cell morphology (indicated with white arrow heads) at the site of stent struts. Exposed struts show platelet, plasma (pla) and scattered inflammatory cell (infl) deposition. In contrast, endothelial cells covering the stent struts of the BMS control show cobblestone structure (marked by asterix), a morphological indicator of contact-inhibited, static endothelial cells. CD31/PECAM-1 immunostaining (green fluorescent signal at cell-cell borders) is detected by confocal microscopy and confirms the presence of well-formed intercellular junctions in BMS control, as a marker of cell maturity, whereas this staining is almost absent in DES.
HISTOPATHOLOGICAL ASSESSMENT BY LIGHT MICROSCOPY
At 28 days following stent implantation percent stenosis was significantly suppressed for all three DES compared to BMS control (Table 2). Neointimal thickness was similar among the DES and differed significantly from BMS control (Table 2). Fibrin deposition was significantly greater in the DES compared to BMS control. In terms of paired comparison, EES differed significantly from SES and ZES in the relative number of struts with fibrin deposition and showed the highest fibrin score and highest percentage in relative fibrin area within the neointima among the different DES (Table 3, Figure 3).

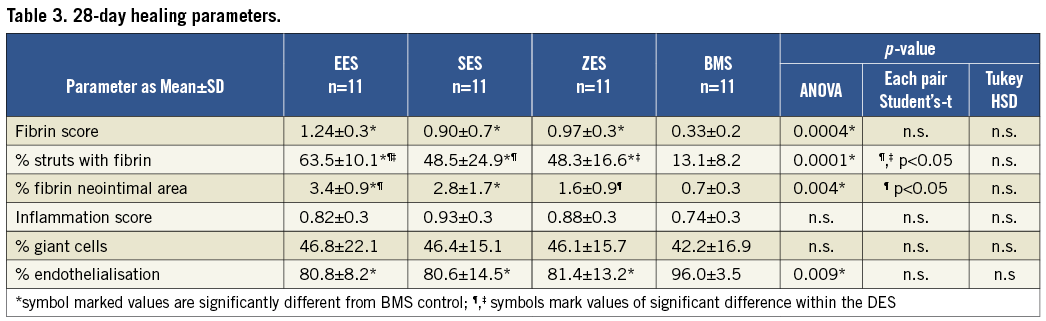
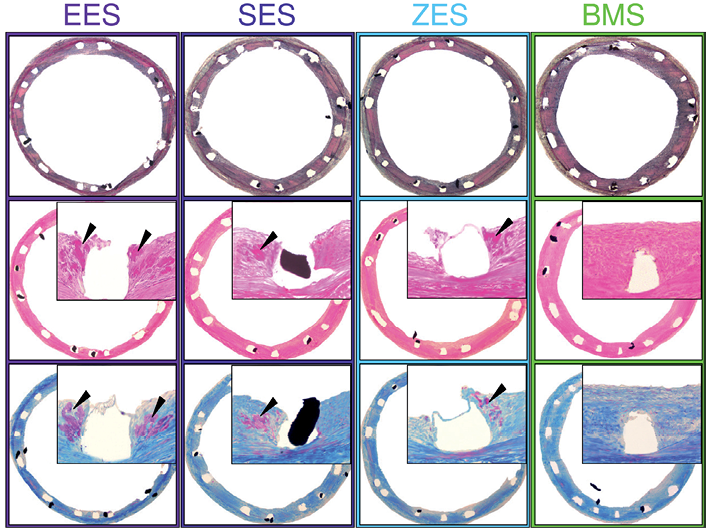
Figure 3. Representative histology sections 28 days after stenting. Movat’s pentachrome (upper images), H&E (middle images) and Carstairs (lower images) stained sections (x40) of the EES, SES, ZES and BMS control. Inserts show high-power images (x200) of the peri-strut areas. Fibrin deposition is marked by black arrow heads.
Discussion
The current study aimed to investigate comparative biological vascular healing responses following implantation of DES eluting different sirolimus derivatives from otherwise identical VISION™ stents (Abbott Vascular) coated with polymer (fluorinated copolymer). The study findings are summarised as:
DES releasing sirolimus, everolimus and zotarolimus from a fluorinated copolymer show a substantial delay in arterial healing, documented by decreased rates of re-endothelialisation and increased fibrin deposition compared to uncoated BMS control at two different time points post-stenting (14 days and 28 days, respectively).
Among the different DES used, there was no difference with respect to re-endothelialisation, either quantitatively or morphologically at 14 days and 28 days following stent implantation.
All DES studied provoked a significant reduction in in-stent neointimal growth compared to uncoated BMS control in a healthy rabbit model at 28 days following stent implantation.
Everolimus-eluting stents displayed greater fibrin accumulation in the peri-strut regions compared to sirolimus-eluting and zotarolimus-eluting stents at 28 days.
RELEVANCE IN CONTEXT WITH PREVIOUS PRECLINICAL FINDINGS ADDRESSING DES RE-ENDOTHELIALISATION
A previous comparator analysis of arterial healing following implantation of the four FDA-approved polymeric DES platforms performed in the rabbit iliac model showed evidence of differential arterial healing patterns across the stents tested6. In particular in terms of endothelial surface coverage, there were marked differences in re-endothelialisation above struts with 64.0±27.5% for XIENCE V®, 30.2±14.2% for Endeavor® (Medtronic, Minneapolis, MN, USA), 26.8±15.8% for TAXUS® and 6.4±4.2% for CYPHER™ (Cordis/Johnson & Johnson, Miami Lakes, FL, USA) at 14 days after stent implantation. A parallel series of tests on markers of endothelial function showed that trends in platelet endothelial cell adhesion molecule-1 (PECAM-1) expression –a glycoprotein involved in endothelial cell-to-cell contact and a marker of cell maturation– were different across DES groups, with XIENCE V® showing greater PECAM-1 expression compared to Endeavor® and CYPHER™ at 14 and 28 days. A similar study utilising the same commercially available DES (except for TAXUS™) analysed the degree of endothelial coverage at 28 days in the atherosclerotic rabbit model10. Here, in line with the healthy animal study, the lowest endothelial stent coverage was detected in the CYPHER™ stent (22±12%) differing significantly not only from XIENCE V® (66±18%) and bare metal control (87±17%) but also from the Endeavor® (70±24%). On the contrary, the other DES did not show significant differences compared to BMS. Furthermore, this study showed that only the Endeavor® reached the level of the BMS for the expression of endothelial nitric oxide synthase (eNOS) differing significantly from XIENCE V® and CYPHER™. The comparison of these two studies with regard to stent re-endothelialisation show consistency only for the CYPHER™, while the new-generation DES seem to alter their histopathological response following implantation in the atherosclerotic animal model at this dedicated time point. However, importantly, the DES platforms tested in these previous studies differed not only in terms of the chosen animal model but also in each of the major stent platform components, namely stent backbone, polymer coating and eluted drug. Consequently it remains unclear which of these individual device components contributed relevantly to the differential re-endothelialisation responses observed. In the current study we addressed the contribution of the eluted drug and assessed whether the sirolimus derivative used on these stents has a differential impact on quantitative and morphologic re-endothelialisation and other markers of vascular healing. We chose the healthy over the atherosclerotic rabbit model as it had been recognised as sensitive to show marked differences in re-endothelialisation among DES at the 14-day time point6,11. So far, no analysis has been performed to determine a sensitive sampling time point with regard to the assessment of re-endothelialisation in the atherosclerotic model.
We found that among the different derivatives used for drug elution there were no significant differences in semi-quantitative re-endothelialisation of the stent strut surface. The endothelial cells exhibited the same morphological characteristics indicative of an immature endothelial cell phenotype. Notably, the DES constructs used in this study were designed to provide uniform release kinetics for the different limus drugs used, which resulted in a total drug load of 40 µg. Even though the applied drug load was capable of suppressing stent re-endothelialisation within all limus drugs at 14 days, the data cannot be extrapolated to the commercial available systems as their drug concentrations are reported to be higher.
The findings of the study suggest that not the choice of the limus drug but the concomitants of stent design, namely drug load, release kinetics and underlying matrix mainly influence the pace of stent re-endothelialisation
IMPACT OF THE ANTIPROLIFERATIVE COMPOUND ON VASCULAR HEALING
To date, two different classes of highly lipophilic drugs have been successfully employed on DES platforms in order to inhibit smooth muscle cell proliferation: (i) drugs of the “limus” family of immunosuppressive agents binding to FK506 binding protein (FKBP12) –including sirolimus, zotarolimus and everolimus and (ii) paclitaxel, a chemotherapeutic agent binding to ß-tubulin.
One of the limitations of the current study is that paclitaxel-eluting stents could not be produced with the manufacturing process employed. Therefore, the comparative impact of an antimitotic drug suppressing neointimal growth as compared to sirolimus derivatives could not be evaluated in the current experimental setting and the general findings arising from our study cannot be extrapolated to DES releasing antiproliferative compounds other than sirolimus derivatives.
This is the first study that showed a greater induction of fibrin deposition in everolimus-eluting stents compared to DES releasing other contemporary sirolimus analogues. To substantiate this finding, we carefully sought to assess fibrin deposition not only by judging the amount of fibrin on the well acknowledged score basis9,12 but also by quantifying the area of peri-strut fibrin relative to the neointimal area. It has been reported that sirolimus derivatives have the potential to induce pro-coagulatory pathways directly, as well as to induce tissue factor expression in a TNF alpha-dependent manner13. Importantly, the increase in tissue factor expression was shown to be similar among the different limus drugs used in vitro. In line with this, most preclinical studies evaluating the vascular effects of limus-eluting DES were able to document a significant increase in peri-strut fibrin deposition, which was substantially prolonged compared to BMS control14,15. Additionally, delayed endothelial regrowth is associated with enhanced thrombogenicity and consequently leads to higher fibrin accumulation in the healing arterial wall. This is an observation that also persists beyond the time point of complete stent re-endothelialisation and therefore renders fibrin a marker of delayed vascular healing11. This is reflected by the study data, as the EES showed lowest endothelial coverage at 14 days with highest fibrin deposition at 28 days, whereas the ZES showed highest endothelial coverage and lowest fibrin deposition. On the other hand, EES was able to catch up with respect to re-endothelialisation at 28 days, while greater fibrin deposition remained. As a consequence of these findings, it is likely that in this particular animal model even small decrements in stent re-endothelialisation may result in substantial increases in peri-strut fibrin deposition as a marker of delayed healing and stent thrombogenicity. As the study design did not include drug content measurements in vivo, we were not able to evaluate whether the observed differences may be related partly to differences in the temporal retention to the vessel wall. Further dedicated preclinical investigation will be necessary to confirm these findings.
IMPACT OF THE POLYMER
In a recent study comparing the vascular effects of polymer-coated sirolimus-eluting stents against polymer-free biolimus-eluting stents, the latter were shown to exhibit diminished inflammatory and fibrin responses at 180 days following implantation in a porcine coronary model without impairment of antirestenotic efficacy16. These findings support the observation from clinical studies which suggest that polymers may also contribute to impaired vascular healing17,18. Despite the fact that the influence of fluorinated copolymer matrix on re-endothelialisation was not addressed in the present study, a prior study found no delay in endothelialisation for polymer-coated stents compared to BMS19. Since a similar polymer was used in the current study, it is likely that the impact of the fluoropolymer on re-endothelialisation is limited. However, the induction of long-term inflammatory responses with polymer-coated stents may still be relevant and remains to be fully investigated, especially in long-term studies.
CLINICAL RELEVANCE IN THE CONTEXT OF THE STUDY FINDINGS
All limus drugs eluted from DES have proven effective in the inhibition of neointimal hyperplasia after stent implantation compared to bare metal stent counterparts20-22. However, there is on-going discussion as to whether the limus drug itself may be the major responsible component for the delayed vascular healing leading to adverse events such as stent thrombosis. The findings of this study, along with the comparison to earlier preclinical work with commercial DES6,10, indicate that the limus drug may have a differential influence on re-endothelialisation and subsequently on delayed vascular healing but that these drug effects are altered to non-relevance, firstly due to varying stent designs, and secondly by the underlying plaque morphology. This becomes particularly evident for the CYPHER™ DES. In this study sirolimus displayed intermediate healing characteristics to everolimus and zotarolimus when eluted from the same stent platform, while elution from the first-generation CYPHER™ platform led to a substantial reduction in stent re-endothelialisation and the highest amount of neointimal fibrin deposition, as shown in the previous atherosclerotic animal study10.
With respect to the clinically reported findings of the everolimus-eluting XIENCE V® stent our data support the notion that everolimus displays an enhanced antiproliferative potential, as fibrin deposition was greatest in the EES group followed by SES and ZES. Consequently, everolimus may achieve the same antiproliferative potency as sirolimus and zotarolimus when applied in lower concentrations. Among the commercial limus DES, XIENCE V® is loaded with the lowest drug concentration (100 µg/cm² compared to 140 and 160 µg/cm² reported for CYPHER™ and Resolute® [Medtronic], respectively). In line with this notion, clinical trials and meta-analysis have shown that EES is associated with equal efficacy and a superior safety profile as compared to other commercial limus DES utilising higher drug loads23,24.
STUDY LIMITATIONS
The current study aimed to investigate the differential biological effects of contemporary sirolimus analogues as one variable of DES design. DES constructs were fabricated to have a uniform stent backbone, polymer and drug load able to provide similar drug release kinetics. Therefore, the results of this study cannot be extrapolated on a 1:1 basis to commercial DES, and due to the limited sample size for the in vivo study additional in vitro analysis could not be performed. Furthermore, minimal variability in coating procedures might have resulted in relevant discrepancies of local tissue drug kinetics, which were not assessed in the present study and therefore cannot be ruled out. Nevertheless, the study was designed to find the value of one variable (drug) in stent design and performance with regard to re-endothelialisation and tissue healing.
In the presence of a non-diseased animal model, the variability in drug retention within the intimal tissue is probably minimal and therefore holds potential to reflect adequately the differences in endothelialisation arising from the selected antiproliferative compounds. However, the impact of these compounds in the presence of atherosclerotic change11 and focal thrombus formation25 may vary substantially and remain unpredictable.
Conclusion
In the current study we demonstrated that sirolimus and its derivatives have similar effects on endothelial regrowth and neointimal thickening. However, greatest fibrin deposition was observed with the everolimus-eluting stents at 28 days in the absence of an incremental delay in stent endothelialisation.
Funding
This study was financially supported by Abbott Vascular, Santa Clara, CA, USA.
Conflict of interest statement
At the time of study performance S. Chin Quee was an employee of Abbott Vascular. R. Virmani is a consultant to Abbott Vascular. The other authors have no conflicts of interest to declare.

