Abstract
Aims: Drug eluting stents (DES) are under scrutiny for late stent thrombosis. Impaired re-endothelialisation is proposed as an explanation but coronary and peripheral-artery models disagree. We assessed physical and functional endothelial restoration within bare (BMS), paclitaxel, sirolimus and tacrolimus eluting stents and the distal microvasculature in porcine coronary arteries.
Methods and results: Endothelium within and distal to DES and BMS was assessed for stent-strut endothelial-restoration (five days) and endothelial-function (five, 28 days, by eNOS and vWF expression) and by in vitro microvascular function. There were no significant differences (P=0.3) in stent strut endothelial-restoration at five days between DES (76-90%) and BMS (95%). However, the microvasculature distal to PES showed a decreased NO bioavailability at five days, which improved at 28 days. Within the stent, however, PES still showed a reduced eNOS expression at 28 days (P≤0.002).
Conclusions: DES in porcine coronary arteries show no significant early differences in re-endothelialisation as compared to BMS. However, PES did affect endothelial function both within and distal to stents. These results extend the concept of delayed healing in DES to include delayed restoration of function rather than endothelial presence as a possible explanation for late unwanted sequelae. Microvascular dysfunction does not predict in-stent delayed endothelialisation in this model.
Introduction
Sirolimus-eluting stents (SES) and paclitaxel-eluting stents (PES) have markedly reduced the rate of in-stent restenosis and late lumen loss compared to bare-metal stents (BMS), resulting in a significantly reduced need for repeat revascularisations.1-3 Enthusiasm for this technology has been dampened by concerns about late thrombosis, an event often with serious clinical consequences.4,5 Aside from rarely occurring delayed hypersensitivity to DES constituents, impaired re-endothelialisation has been suggested as the likely cause of late DES thrombosis based on one clinical autopsy study6 and experimental overlapping DES implantation in denuded and injured rabbit peripheral arteries.7,8 Although data from denudation and overlap models cannot be dismissed, they do not represent the full spectrum of the clinical arena. Indeed, there is consistent evidence pointing towards late endothelial dysfunction as the main derangement after DES. This was shown both experimentally and clinically as impaired responses to endothelial dependent vasodilators or exercise.9-11
Combining assessment of in-stent re-endothelialisation with endothelial-function of the newly formed neointima and distal vasculature has not been reported. This combined assessment is important as altered endothelial-function could be the result of both dysfunction as well as absence of endothelium. Discriminating between these two is, however, difficult to do in patients and a correlation between these two parameters might theoretically be a diagnostic tool for endothelium within the stent.
Predicting which animal model mimics the human situation the closest, is difficult and may well differ between drugs due to species differences in sensitivity. Even the choice for healthy, injured or atherosclerotic models will affect outcome. While in atherosclerosis general endothelial-function may be affected, there is no indication it delays endothelial-regrowth.12,13 We therefore studied the effects of three distinctively different DES on early endothelial-regrowth (five-days follow-up) as well as early and late endothelial-function (five and 28 days follow-up) both with the stent and in the distal coronary microvasculature (i.e., not the epicardial coronary artery) in normal, non-atherosclerotic swine.
Materials and methods
Stents
Four different stent types with a length of 12-13 mm (diameter 3.0 and 3.5 mm) were studied: 1) 15 bare metal controls (BMS, Kaneka corporation, Osaka, Japan), 2) 25 tacrolimus eluting stents (TES, 0.9 µg/mm2, Kaneka corporation, Osaka, Japan) 3) 25 sirolimus eluting stents (SES, 1.4 µg/mm2, Cordis, Johnson & Johnson, Warren, NJ, USA) and 4) 25 Paclitaxel eluting stents (PES, 1 µg/mm2, Boston Scientific, Natick, MA, USA).
Polymers
The TES coating consists of a fully degradable polylactide-polyglycolide polymer (PLGA), with a degradation rate ≥90 days.14,15 SES is coated with biostable poly-ethylene-vinyl-acetate-poly-butylene-methyl-acetylate (PEVA-PBMA), PES is coated with a biostable styrene-b-isobutylene-b-styrene (SIBS).16
Drugs
The intracellular receptors of tacrolimus are the FK binding proteins (FKBP, including FKBP12). The tacrolimus-FKBP complex binds to the calcineurin-calmodulin complex.17 Tacrolimus effects on endothelium includes inhibition of expression of endothelial adhesion molecules, and reduction of free radical and inflammatory cytokine release.18,19 Sirolimus also binds to FKBP12, but the Sirolimus-FKBP complex then inhibits mTOR20, halting cell cycle progression from G1 to S, as well as vascular cell migration. Paclitaxel inhibits depolymerisation of tubulin, thereby arresting cellular replication and inhibiting migration of vascular smooth muscle cells.21,22
Animals and preparation
Experiments and animal preparation were performed in farm-bred swine (Yorkshire-Landrace, 30-35 kg) as previously described.23,24 The study was approved by the animal ethics committee of the Erasmus University, Rotterdam and complied with the “Guide for the care and use of laboratory animals” (NIH publication 85-23). In short, animals were pretreated with 300 mg acetyl salicylic acid (ASA) and a loading dose of 300 mg of clopidogrel (Plavix; Sanofi Aventis, Gouda, The Netherlands) one day prior to the procedure. After induction of anaesthesia and connection to the ventilator, antibiotic prophylaxis was administered by an intramuscular injection of streptomycin, penicillin procaine (0.1 mg/kg). An introducer sheath was placed in the carotid artery for arterial access. A dose of 250 mg ASA and 10.000 i.u. heparin was administered (i.a.).
Stent implantation and angiography
Stent implantation was performed as described before.23,24 Under guidance of quantitative angiography (QCA, CAAS II; PIE Medical, Maastricht, The Netherlands), arterial segments of 2.5-3.2 mm in diameter were selected in each of the coronary arteries. Stents were placed with a balloon-artery (B/A) ratio of 1.1 without prior injury. Animals received one stent per coronary artery, according to a predetermined randomisation scheme for stent type. After the final angiogram, animals were allowed to recover from anaesthesia and returned to animal care facilities for postoperative recovery. During follow-up, 300 mg ASA and 75 mg clopidogrel were administered daily. Only in swine followed for 28 days, follow-up angiography was planned prior to euthanasia. This was omitted for the five day group to prevent catheter induced injury.
Tissue harvesting
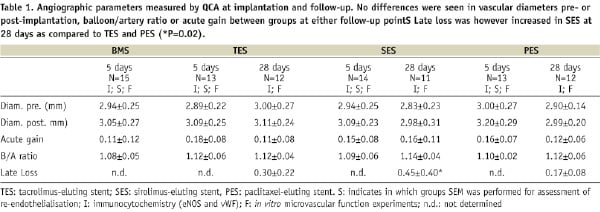
Animals were euthanised by an overdose of pentobarbital. Hearts were pressure fixed in situ in preparation for histology.24 In case of in vitro vasomotor tone experiments, fixation was preceded by excision of small myocardial samples from the distal perfusion area of the stent for the isolation of the vessels (Table 1).
Endpoints
Completeness of the endothelial layer by scanning electron microscopy (SEM) and endothelial function by eNOS- and vWF-expression were studied at five days. Endothelial presence, histology and endothelial eNOS- and vWF-expression were studied by light microscopy at 28 days. In a subset of the animals (Figure 4), distal vasomotor tone was assessed by in vitro functional tests at five and 28 days.
Scanning electron microscopy
After fixation with 2.5% glutaraldehyde in 0.15 mol/l cacodylate buffer, the arteries were further processed as previously described.25 From photographs, the stent struts were traced, the endothelium marked, and the percentage endothelial coverage of the stent struts quantified using computer assisted planimetry, (Clemex vision PE; Clemex Technologies Inc., Longueuil, Canada).
Histology
The arteries were processed for plastic embedding.23,26 Sections were collected at three levels (proximal, mid and distal) of the stented segments. The sections were stained with haematoxylin-eosin and resorcin-fuchsin (elastin stain) for quantitative and qualitative analysis.
Morphometry
Analysis of neointima and injury was performed as described before25. As at 5-days all stents were covered by thrombus and granulation tissue containing inflammatory cells, inflammatory scores were only determined at 28 days, on HE stained sections, and given as the ratio of inflamed over total number of struts at each level (proximal, mid and distal) and averaged per stent.
Immunocytochemistry
The complete circumference of the stented vascular segment was assessed semi-quantitatively for eNOS (sc-654; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and von Willebrand Factor (vWF) (A0082; DakoCytomation, Copenhagen, Denmark). Scores were assigned as: 0=0% positive circumference, 1=0-10%, 2=10-40%, 3=40-70%, 4=70-90%, 5=90-100%.
In vitro microvascular function
Upon sacrifice, microvascular coronary arteries (~300 µm diameter) were dissected free from the myocardial tissue in the perfusion territory of the stent. Experiments were performed as previously described.27-29
In brief the arteries were cut into segments of ~2 mm length and mounted in a Mulvany wire myograph (DMT A/S, Aarhus, Denmark). Following 30-minutes stabilisation, the optimal internal diameter was set to a tension equivalent of 0.9 times the estimated diameter at 100 mmHg effective transmural pressure.30 Vascular responses were measured as changes in isometric force. Segments from the same vessel were randomly assigned to a different experimental protocol, such that in each perfusion territory all measurements were performed. After stabilisation, the vessel segments were exposed to 100 mM KCl to determine the maximal contractile response to KCl. Upon wash-out of KCl, the segments were allowed to equilibrate for 30 minutes. The endothelium-dependent relaxation to bradykinin (10–10-10–6 M) and endothelium-independent relaxation to the exogenous nitric oxide (NO) donor, S-nitroso-N-acetylpenicillamine (SNAP, 10–7-10–6 M) was recorded upon preconstriction with the thromboxane analog U46619 (1nM). In order to test the contribution of NO-mediated mechanism to the bradykinin-induced relaxation, the concentration–response curve for bradykinin was constructed after 30 min. incubation with the eNOS inhibitor N-Nitro-L-Arginine methyl ester (L-NAME, 10–4 M), in a separate set of vessels. Data of six historic control vessels from two animals receiving a BMS for 28 days were used for comparison in the vascular function study.
Statistical analysis
All data are given as mean ±SD except for in vitro microvascular function which is given as mean ±SEM. Histology and QCA were analysed with SPSS version 16, (SPSS Inc., Chicago, IL, USA). Intergroup differences were assessed using one-way ANOVA followed by post-hoc analysis with a Bonferroni correction in case of statistical significance. P<0.05 was considered statistically significant. In vitro relaxant responses to bradykinin and SNAP are expressed as a percentage of the contraction to U46619 and were analysed using Stat View (version 5.1). Statistical significance was established by a two-way ANOVA followed by post-hoc analysis with a Bonferroni correction in case of statistical significance. P<0.05 was considered statistically significant. Further testing was performed using linear regression analysis (SPSS). For QCA the main model parameters consisted of follow-up time, balloon-artery ratio and stent type. For morphometry and immunocytochemistry the model parameters also included the morphologic injury score and inflammation score. For SEM analysis the model parameters were procedural characteristics (B/A ratio, acute gain) and stent characteristics (drug, design, metal to surface ratio, strut width).
Results
Quantitative angiography showed no differences in diameters between pre- or post-implantation or stent/artery at either follow-up time (Table 1). Late loss at 28 days was not different between TES (0.3±0.2) and PES (0.2±0.1), only SES showed a significant late lumen loss (0.5±0.4 mm, p=0.02 versus PES by ANOVA), as a result of inflammation. Regression analysis did not yield a significant predictive model (ANOVA p=0.6).
Scanning electron microscopy
Data (Table 2), indicate that as early as 5-days the stent struts were covered with endothelium to a large extent.
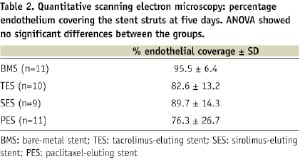
There were no significant differences in re-endothelialisation between DES and BMS (one way ANOVA p=0.3) (Figure 1), indicating that the drugs did not inhibit re-endothelialisation. Regression analysis (ANOVA p=0.02) confirmed that the drug did not predict re-endothelialisation. Only stent-artery ratio (p=0.03), stent design (p=0.02) and metal to surface ratio (p=0.02) were independent predictors of re-endothelialisation. The predictive value of the model was, however, rather low (r2=0.4).
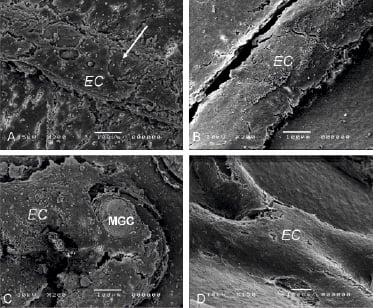
Figure 1. Scanning EM of the endothelial layer overlying stent struts in Bare (A), TES (B), SES (C) and PES (D), showing extensive endothelial (EC) coverage of the struts. Areas devoid of EC (arrow) often show presence of macrophage giant cells (MGC).
Histology
At 5-days all stents showed immature granulation tissue in varying stages of development (Figure 2).
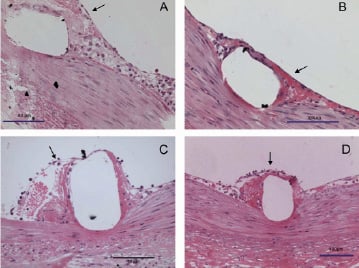
Figure 2. Light microscopy images of stent struts covered with immature granulation tissue showing scarce cellular infiltration and loose endothelium (arrows) at 5-days following implantation of BMS (A), TES (B), SES (C) and PES (D).
Consistent with SEM, stent struts were already endothelialised to a large extent. Lack of endothelium often coincided with presence of macrophage giant cells. Leukocytes were often found adhering to the endothelium. In general, TES showed a response similar to BMS. This granulation tissue was more mature in terms of inflammation and colonisation with smooth muscle (SMC)-like cells than in PES, while SES generally showed the most immature healing response.
At 28 days re-endothelialisation was virtually complete in all groups, and in general the neointima showed a mature and organised appearance (Figure 3).
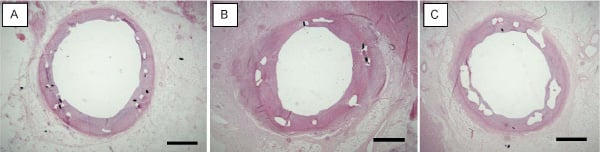
Figure 3. Three examples of coronary arteries at 28-days following stenting with TES (A), SES (B) and PES (C), showing complete coverage with an endothelialised fibrocellular neointimal thickening. HE-stain. Bar=1000 µm
Each drug, however, had a specific appearance: PES showed apoptotic cells and mitotic figures in both neointima and media. SES showed luminal and adventitial eosinophilic granulocytes and abundant fibrinoid in the intima-media border zone. TES showed acidic proteins in the intima-media border zone, possibly proteoglycans.
Morphometry
Data is summarised in Table 3.
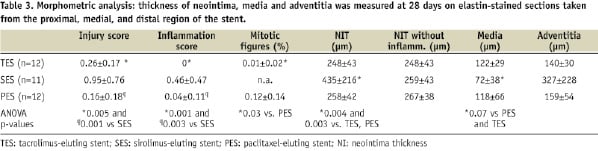
At 5-days, all stents tend to show thrombus being incorporated and cleared by granulation tissue.
The damage to the tissue induced by histological sectioning overlying the struts was however too great to yield reliable quantitative data and morphometry of the intima was not performed. At 28-days, SES induced a considerable inflammatory response in a number of animals which increased intimal thickening considerably. We therefore compared the results also in the SES subgroup without inflammation. This showed that neointima thickness (NIT) was not significantly different between the groups upon exclusion of stents with severe inflammation. Injury and inflammation score, when analysed separately as model parameters in regression analysis, were both strong predictors of NIT (p<0.0001). However, when used simultaneously they were not independent. Endothelial-function (eNOS, vWF) was not an independent predictor of NIT.
Immunocytochemistry
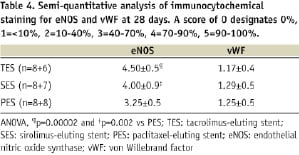
This data is summarised in Table 4.
At 5-days all DES contained eNOS positive endothelium (Figure 4).
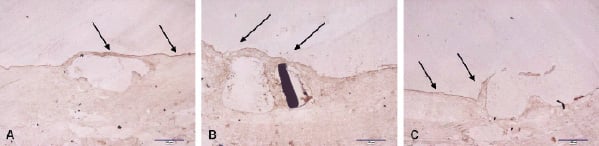
Figure 4. eNOS staining of porcine coronary arteries at 5-days following stenting with a TES-(A), SES (B), and PES (C) stent. The brown staining, both on the stent struts and between the stent struts, denotes eNOS positivity (arrows).
The damage to the tissue overlying the struts was however too large to yield reliable quantitative data. At 28 days regression analysis of eNOS expression (ANOVA p=0.001, R2=0.6) indicated that eNOS expression was significantly less in PES as compared to TES and SES (See Table 4, p<0.002). Inflammation had no effect on eNOS expression (p=0.18), only injury score was an independent negative predictor of eNOS expression (p=0.006). In all groups, the number of cells expressing vWF was very low (score ~1-1.3), showing no significant differences between groups.
In vitro vasomotor tone
Data show that bradykinin induced similar levels of endothelium-dependent vasodilation in the microvasculature, independent of stent type and follow-up time. Therefore the five and 28 day data on control bradykinin responses were pooled (Figure 5A). Distal to BMS, TES and SES, eNOS-blockade resulted in a significant impairment in bradykinin-induced dilation both at five and 28 days (Figures 5B, C). In the PES area, however, at 5 days L-NAME had little effect on the dilation indicating a reduced contribution of NO to this response (Figure 5B). This lack of NO-bioavailability was not correlated to re-endothelialisation within that stented segment (Regression analysis, ANOVA p=0.4). The NO contribution did recover at 28 days (Figure 5C). Endothelial-independent dilation to SNAP was similar for all stents and follow-up times (Figure 5D).
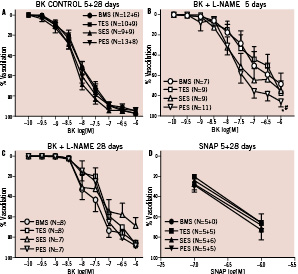
Figure 5. Endothelial-dependent dilation to bradykinin and –independemt to SNAP in small coronary arteries distal to BMS, TES, SES and PES. No difference was seen between control curves at 5 and 28 days, therefore, data were pooled (Fig. 5A). eNOS blockade by L-NAME resulted in a significant reduction in BK-induced dilation. However, at 5 days, the effect of eNOS blockade was impaired distal to PES (Fig. 5B, * P<0.05 vs BMS, # P<0.05 vs TES) as compared to the other stents. No difference was seen between stents at 28 days (Fig. 5C). No difference was seen in SNAP responses at 5 or 28 days (5D).
Discussion
We set out to assess differences in re-endothelialisation and endothelial function after implantation of TES, SES, PES and BMS in normal swine coronary arteries. We found that DES demonstrated 76-95% in-stent endothelialisation at five days after implantation. This was not statistically different from BMS. In-stent endothelial function as assessed by eNOS expression, was already demonstrable at 5-days, and almost complete for TES and SES at 28 days after implantation. PES, however, showed a significantly reduced in-stent eNOS expression at 28 days. Consistent with this finding we demonstrated a transient impairment in the NO contribution to endothelial-dependent relaxation in the microcirculation distal to PES, suggesting abnormal functioning eNOS, which was not correlated to re-endothelialisation.
The current study demonstrates that the functional impairment of the endothelium can be shown even in presence of apparent structural integrity. This finding does not automatically allow to dismiss the idea that in atherosclerotic arteries there can be instances where the endothelial regrowth is incomplete following DES placement. The clinical sequelae of DES are probably resulting in individual cases from either structural or functional compromise of cellular coverage, or both acting in concert in some other cases.
Re-endothelialisation
The current view is that DES impair re-endothelialisation, supported by data from a single institution reporting one human autopsy study (coronary), and non-coronary DES implantation in rabbit iliac arteries.6-8 Clinical angioscopy and OCT studies imaging DES also suggest delayed endothelialisation31-34. But because the resolution of angioscopy and OCT are insufficient to detect endothelium on stents, we regard those studies as not contributing to the evidence.
Our results do not support the concept of delayed endothelialisation as we show no significant difference in re-endothelialisation between DES and BMS as early as five days. This is compatible with previous animal studies. In a study by Klugherz et al in a rabbit iliac artery model, no evidence of delayed endothelialisation was noted with 28-day SES compared with polymer-coated stents or BMS.35 In addition, in SES and PES a similar degree of re-endothelialisation (>75%) was reported in porcine coronary models36-38. Thus, overall, the support for the delayed re-endothelialisation concept is equivocal.
Factors influencing endothelialisation
In order to assess the influence of stent and experimental conditions on re-endothelialisation, we performed regression analysis with stent characteristics (design, drug, strut width, metal to surface ratio) and implantation characteristics (balloon-artery ratio and acute gain) as model parameters. Design, metal to surface ratio, and balloon-artery ratio were independent predictors, while drug, strut width and acute gain were not. However, the predictive value of the model was rather low (R2=0.4), suggesting that other factors, like the kinetics of drug elution and retention in the vascular wall should be examined in order to fully explain the data. There was no significant correlation between in-stent re-endothelialisation and distal microvascular dysfunction.
A recent study indicates that in rabbits, DES do inhibit re-endothelialisation in previously denuded and injured peripheral arteries at “early’ time points (14 days).8 A comparison with previous data from the same group indicates that endothelial regrowth at 28 days varies considerably for some (but not all) drugs and may be subject to experimental conditions.7 In addition, a rabbit is much smaller than a swine or a human, and there are large species differences of drug stability in plasma and cellular sensitivity in culture.39-41 Therefore, predicting which animal model mimics closest the human situation is difficult and may well differ between drugs.
Endothelial function
While a large percentage of endothelial coverage at early stages is considered important by many, it should be clear that this does not guarantee full functional integrity of the endothelium.24 In a similar regression model as described above, in-stent eNOS expression was negatively predicted by morphologic injury score (acute implantation damage in combination with chronic irritation by the stent) while inflammation was not an independent predictor, nor any angiographic parameter, indicating that chronic vascular damage plays an important role in the functionality of the endothelial layer after stenting. The reduced in-stent eNOS expression observed in our study in PES at 28 days, when the stent is almost completely re-endothelialised, shows that presence of endothelium is not synonymous to endothelial-function. Indeed, late in-stent endothelial dysfunction may lead to increased platelet and leukocyte adhesion which could be one of the possible mechanisms responsible for the increased risk for late stent thrombosis reported in patients receiving PES.42,43
Microvascular vasomotor function
It has been shown clinically that DES affect vascular function immediately distal to stents9,10,44. Our in vitro data indicate that early following stent implantation, these effects extend also into the distal microvasculature. We used bradykinin, a clinically relevant agent45 as an alternative to acetylcholine as porcine coronary arteries have limited presence of muscarinic receptors and acetylcholine therefore does not induce sufficient vasodilation46-48. In the small vessels distal to PES we found a significant reduction of the NO contribution to the bradykinin-induced dilation at 5-days follow-up. This was not due to a reduced smooth muscle cells sensitivity to NO, as the response to the exogenous NO donor SNAP remained unchanged. In healthy porcine coronary arteries bradykinin-induced dilation is mediated only by NO or EDHF (endothelium-derived hyperpolarising factor)28. Our data do indicate that EDHF fully compensated for the reduced NO production in PES. Interestingly in-stent re-endothelialisation in PES was not correlated to distal microvascular dysfunction in PES, nor any other parameter studied in regression analysis. It is unclear what is needed to predict microvascular dysfunction.
Although PES still showed a reduced in-stent eNOS expression at 28 days, no overt endothelial dysfunction was seen in the distal microvasculature at this time. Thus distal endothelial NO production appeared to recover faster than within the stent itself. Our in vitro observations are consistent with Togni44 who also showed an improvement in endothelial function over time following PES implantation in patients.
Conclusion
In this study, all DES and BMS showed similar early re-endothelialisation. While PES showed a significant effect on endothelial function both within the stent as well as in the distal microvasculature, we did not find a correlation between the two phenomena. These results extend the concept of delayed healing in DES to include delayed restoration of function as a possible explanation for late unwanted sequelae. Microvascular dysfunction does not predict in-stent delayed endothelialisation in this model.
Acknowledgements
The technical assistance of I. Krabbendam-Peters, BSc; S.C. Krabbendam, BSc is gratefully acknowledged.

