Abstract
Aims: To evaluate the time-course of vasomotor function and re-endothelialisation after implantation of a novel platinum-chromium (PtCr) abluminal biodegradable polymer-coated paclitaxel-eluting stent (PES, Labcoat Element) in rabbit iliac arteries.
Methods and results: Either PES (n=18) or an identical platform of bare metal stents (BMS, Element, n=18) were implanted in rabbit iliac arteries (six animals per time-point). At 14, 30, and 90 days, acetylcholine- and nitroglycerine-induced vasomotor reactivity at 5-10 mm distal to the stent was measured. Subsequently, the animals were terminated. The stented artery was bisected longitudinally for either SEM or en face CD31 immunochemistry examination. All arteries were patent with normal angiographic flow. Decreased endothelial-dependent vasomotion was found at both 14 and 30 days for PES compared to BMS (p<0.01, respectively); however, these differences resolved by 90 days. Endothelial-independent vasorelaxation was similar at all three time-points. Both SEM and en face staining demonstrated equivalent endothelial coverage on the surface of the stented segments above and between struts at all time-points.
Conclusions: This novel bioabsorbable polymer abluminal-coated PES demonstrated vasomotor function comparable to BMS within three months post-deployment in the rabbit iliac model. Despite indistinguishable endothelial cell coverage on the stent surface between groups, earlier differences in vasomotion were detected: this finding suggests that the timing of restoration vasomotor function lags morphologic endothelial recovery.
Introduction
Development and widespread utilisation of drug-eluting stents (DES) have provided a novel and effective treatment for the reduction of in-stent restenosis, target lesion revascularisation, and clinical symptom recurrence compared to bare metal stents (BMS). However, complications including impaired re-endothelialisation, vasomotor dysfunction, and stent thrombosis seen in earlier generations of DES may overshadow the antiproliferative potential. Additionally, DES require long-term dual antiplatelet therapy, with the attendant morbidities and socio-economic consequences1-7. Therefore, modern DES technology is aimed at balancing arterial healing with therapeutic effects.
The Element stent (BMS; Boston Scientific Corp., Natick, MA, USA) is composed of a thin strut (81 µm) platinum chromium (PtCr) alloy, which has enhanced radiopacity, radial strength and fracture resistance. The Element stent design incorporates a dimensionally uniform pattern of serpentine segments, each with two offset connectors, which reverse the direction of alternate rows to maintain a balance of forces along the stent. This design also improves deliverability and conformability and reduces recoil8. With a duplicate stent platform, the Labcoat Element (PES; Boston Scientific Corp., Natick, MA, USA) contains PLGA bioabsorbable polymer and paclitaxel at a dose of 10 µg/12 mm of stent. The elution of drug is solely abluminal and fully released within 90 days, coupled to the progressive degradation of the PLGA polymer by hydrolysis. Within four months, both drug and polymer have dispersed, leaving a polymer-free BMS and aimed to promote endothelial recovery.
A number of studies have reported first-generation DES-associated vasomotor dysfunction at six to 12 months post stent implantation: this phenomenon is postulated to result from local vessel wall toxicity, inflammation and oxidative stress7,9-11. Therefore, the purpose of this study was to investigate endothelium-dependent and endothelium-independent vasomotor function and re-endothelialisation and to understand the time-course of vascular response to a novel PtCr biodegradable polymer abluminal coated PES in a rabbit iliac artery model.
Methods
ANIMAL AND STENT IMPLANTATION
Animal handling and care followed the recommendations of the National Institutes of Health (NIH) guide for the care and use of laboratory animals and was consistent with guidelines of the American Heart Association. All protocols were approved by the Animal Care and Use Committee and were consistent with the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) guidelines.
A total of 36 stents from two different groups, PES (n=18) and BMS (n=18) were implanted in rabbit (3.5-4.0 kg) iliac arteries. All animals received 1 mg/kg aspirin and 1 mg/kg clopidogrel daily for a minimum of three days prior to the procedure and daily thereafter until termination. All rabbits were fasted overnight prior to the stent implant procedure. Sedation was accomplished by intramuscular injection of ketamine 35 mg/kg and xylazine 5 mg/kg, followed by maintenance inhalant isoflurane via nose cone delivered at a concentration of 0.5-5% in a mixture of O2 and N2O. Electrocardiogram and blood pressure were continuously monitored during stent implantation, angiographic re-study, and vascular function studies. As previously described by our group12, catheterisation was performed through carotid cut-down following heparinisation (200 units/kg). After engaging a 5 Fr multipurpose guide catheter, identical stents (2.75×12 mm) were deployed bilaterally with 12 atm for 15-second inflations, which reached about 1.3:1 stent to artery ratio. After post-deployment a check arteriogram confirmed stent apposition and vessel patency, carotid vessel access was closed, and animals were allowed to return to cages.
IN VIVO EVALUATION OF ENDOTHELIAL FUNCTION
At 14, 30 and 90 days (six animals per time-point), endothelium-dependent vasomotor function at 5-10 mm distal from non-stented reference segments was assessed after infusion of two incremental doses of acetylcholine (Ach, 10–5 and 10–4 mol/L) via the guide catheter just above the iliac bifurcation. Ach was delivered through an infusion pump (Harvard Apparatus, Holliston, MA, USA) at 1 ml/min for three minutes, with precisely five minute intervals between each injection. Endothelium-independent function analysis was assessed by intra-arterial bolus nitroglycerine (NTG, 200 µg). Angiography was performed before and 30 seconds after each drug administration. The animals were then terminated for histologic evaluation.
QUANTITATIVE ARTERIOGRAPHY ANALYSIS
Iliac arteriograms were performed using a digital x-ray system (Allura FD10, Royal Philips Electronics, Amsterdam, The Netherlands) in an anterior-posterior projection at 15 frames/sec. The monoplane view selected provided a clear visualisation of the entire target artery. The distance from the animal to the image intensifier was kept at its closest position. Off-line quantitative coronary measurements were performed using a computer-based system (Xper) by an independent unbiased observer, blinded to the study assignment. The mean luminal diameters of 5 mm segment lengths were calculated at 5-10 mm distal to the stent. Each segment was referenced to a specific anatomic landmark for identification. Responses of the luminal diameters to infusions of acetylcholine and nitroglycerine were expressed as percentages of diameter changes from the baseline prior to infusion.
SCANNING ELECTRON MICROSCOPY (SEM) EVALUATION
The stented arteries were harvested at 14, 30 and 90 days to assess endothelial coverage. Post-bisection, longitudinally-incised, half vessels were processed for scanning electron microscopy (SEM), which were rinsed in sodium phosphate buffer (0.1 mmol/L, pH 7.2) and post-fixed in 1% osmium tetroxide for 60 minutes, followed by serial ethanol dehydration. After critical point drying using liquid CO2, the samples were mounted and sputter-coated with 30-40 nm gold. The entire luminal surface was examined in a JSM-6490LV SEM (JEOL Ltd., Tokyo, Japan), and digital images were recorded to estimate the degree of endothelial cell coverage of the implant. The endothelialised luminal surface at above stent struts and between stent struts was expressed as the percentage of endothelialised areas above and between stent struts/total stented segment surface ×100.
EN FACE IMMUNOSTAINING FOR PLATELET-ENDOTHELIAL CELL ADHESION MOLECULE
Expression of platelet-endothelial cell adhesion molecule (PECAM-1) was used to localise endothelial cells (ECs) on the surface of the stented vessel. The entire opposing bisected longitudinal specimens were evaluated by indirect immunostaining with anti-CD31 antibody (PECAM-1, 1:40 dilution; Dako, Carpentaria, CA, USA). After washing in PBS and blocking in 10% normal goat serum (Jackson ImmunoResearch, Inc., West Grove, PA, USA), the samples were incubated with PECAM-1 for one hour at room temperature. Subsequently, they were washed in PBS again, and incubated for one hour with secondary antibody conjugated to Alexa Fluor647 (1:200 dilution; Invitrogen Corp., Carlsbad, CA, USA) for visualisation. Images were acquired on a laser confocal microscope (Olympus Fluoview FV1000) and captured according to 4 µm step-wise increments between Z-stacks at 200X magnification. Image processing was performed by using Metamorph Offline 7.0.1.3 software. Fluorescent images were overlaid and photo-merged with differential interference contrast images to denote the pattern of endothelial cell coverage over stented surface.
STATISTICAL ANALYSIS
All data were presented as mean±SD unless otherwise indicated. Statistical analysis was performed by STATA version 10.0 (StataCorp, College Station, TX, USA). Angiographic data and endothelial coverage data were evaluated by Student’s unpaired t-test. The mean values of coronary vasomotor responses between the two groups were compared using repeated ANOVA. A p-value <0.05 was considered significant.
Results
There were no complications or mortalities related to stent implantation or anticoagulation treatment at immediate post-procedural, 14, 30 and 90-day follow-ups. The stent was clearly visualised under fluoroscopy. Arteriography showed patent stent arteries with appropriate sizing and normal brisk forward flow.
COMPARISONS OF PES AND BMS ASSOCIATED VASOMOTOR FUNCTION
Endothelium-dependent vasomotion was determined by changes in the distal non-stented reference segments iliac artery diameter from baseline and after drug infusions. Vasodilatory responses to incremental doses of Ach were significantly diminished in PES compared to BMS at the initial two time-points. Mean iliac diameter changes were –0.92±6.37% for PES and 10.50±5.93% for BMS after Ach 10–5 M/ml (p=0.004), as well as 0.80±3.96% for PES and 10.72±6.30% for BMS after Ach 10–4 M/ml (p=0.005) at 14 days. Similar patterns were observed at 30 days, which were –5.08±7.47% for PES and 7.58±4.09% for BMS after Ach 10–5 M/ml (p=0.004), and –4.61±4.30% for PES and 10.17±3.03% for BMS after Ach 10–4 M/ml (p<0.001). However, at 90 days, Ach-induced vasomotor function was seen to recover in PES, to a level comparable to that of BMS. Table 1 is repeated ANOVA analysis of the vessel diameter changes in response to Ach. The mean iliac diameter changes were 3.76±9.53% for PES and 8.79±11.49% for BMS after Ach 10–5 M/ml (p=0.429), as well as 8.49±12.59% for PES and 12.81±10.32% for BMS after Ach 10–4 M/ml (p=0.530). NTG-induced endothelium-independent vasorelaxation was comparable between groups at all three time-points. Figure 1 shows the arteriographic images during vasomotor function test at 30 and 90 days. Figure 2 illustrates the arterial diameter changes in response to escalating doses of Ach and nitroglycerine at 14, 30 and 90 days.
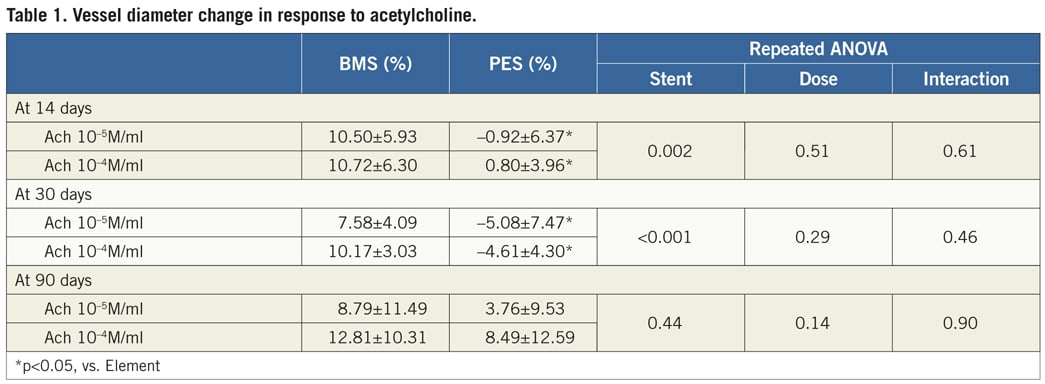
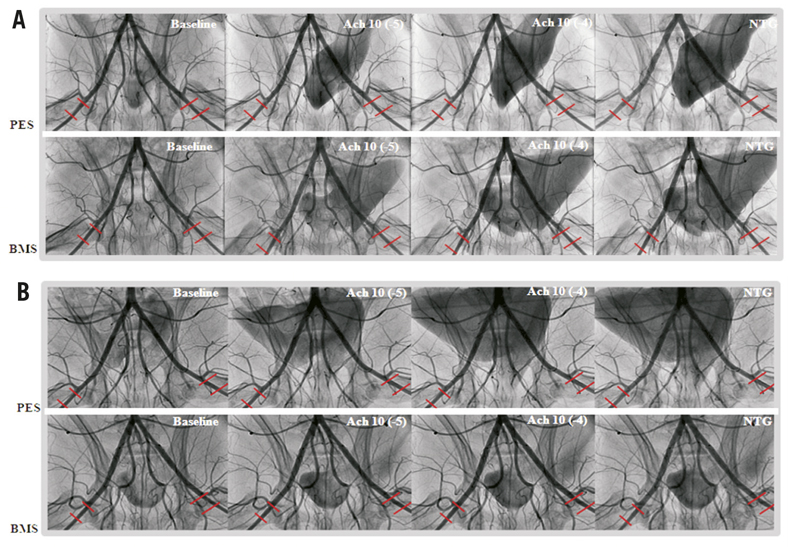
Figure 1. Representative angiographic images for PES (upper panel) and BMS (lower panel) during endothelial function tests at baseline, incremental doses of Ach (10–5 M/ml, and 10–4M/ml), and nitroglycerine 200 µg at day 30 (A) and at day 90 (B). At 30 days, mild vessel spasm in response to Ach was found in distal segments in PES compared to BMS. In contrast, there were no differences between groups at 90 days, indicating recovery of vasomotor function alone with time. Ach: acetylcholine; PES: Labcot Element; BMS: Element.
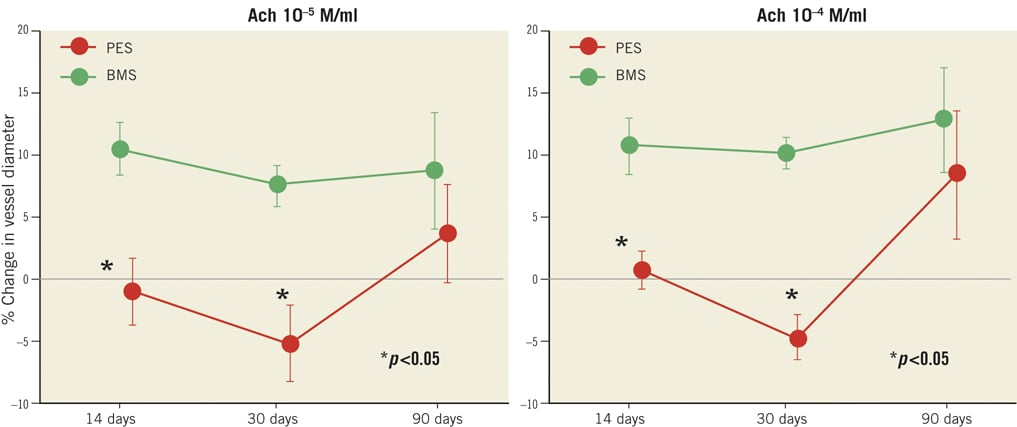
Figure 2. Comparison of the percentage vessel diameter changes between PES and BMS in response to Ach and nitroglycerine 200 µg at all time-points. Endothelial-dependent vasodilatory response to Ach was diminished in PES at 14 and 30 days (p<0.05, respectively), and recovered at 90 days, as compared with BMS. Ach: acetylcholine; PES: Labcot Element; BMS: Element. Data are expressed as mean±SD. *p<0.05 vs. BMS.
ENDOTHELIAL COVERAGE OF STENT SURFACE
No mural thrombi were detected on the luminal surfaces of the stented arteries by either gross or microscopic examination at any time-point. In general, SEM observation revealed intact neointimal coverage of stent struts (SS). The footprint of SS can be visualised and traced at early time-points; however, it became hard to visualise in the BMS group at 90 days compared to 30 days under the same low-power magnification. By contrast, coverage of PES was still discernible at later time-points, indicating a thinner neointimal formation versus BMS. Quantitative SEM showed that the degree of re-endothelialisation was no different at any time-point. The endothelial coverage above and between stent struts was more than 90% for both PES and BMS (Table 2). In regions uncovered by endothelium, focal aggregates of platelets and inflammatory cells were found in both groups under high-power magnification. Figure 3 is representative of SEM images, which showed the time-course of endothelial coverage after PES and BMS implantation in rabbit iliac arteries. Consistent with the SEM findings, the expression of CD31 by immunohistochemistry was similar between PES and BMS in all of the follow-up times. Confluent endothelial cell monolayers were found across the entire stented artery surface. There was no positive staining in the slides if the primary antibody was omitted. Figure 4 is representative of en face PECAM-1 staining, which demonstrated the time-course of endothelial cell staining after PES and BMS implantation in rabbit iliac arteries.


Figure 3. Scanning electron microscopy (SEM) images of the time-course following implantation of both PES and BMS. The stent strut was covered by a layer of neointimal tissue, which was visualised as a sine wave of the footprint. The visibility was greatly decreased in BMS at 90 days in comparison with PES.
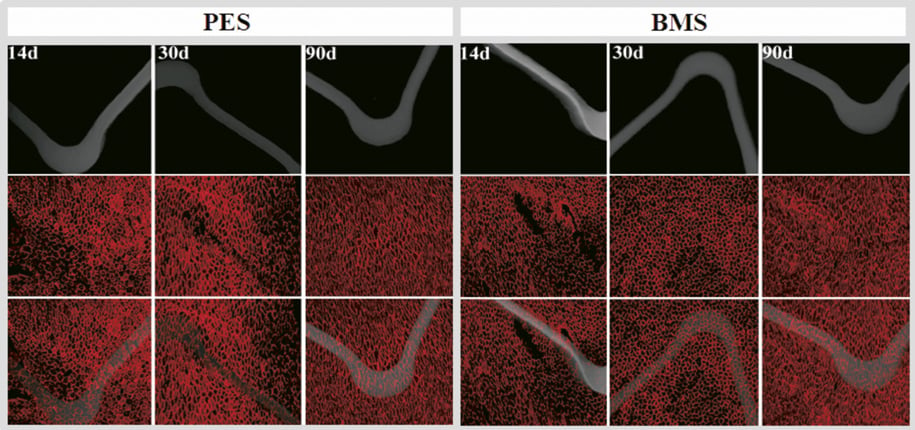
Figure 4. En face PECAM-1 (CD-31) staining, which demonstrated the time-course of endothelial cell coverage after PES and BMS implantation in rabbit iliac arteries. The stent strut was covered by a layer of CD31 positive stained cells, and no difference was distinguished between groups at all time-points. PES: Labcot Element; BMS: Element. Upper row: stent struts only; middle row: CD31 only; lower row: overlapping images.
Discussion
In the present study, a novel PtCr stent coated abluminally with biodegradable polymer PES demonstrated comparable vasomotor function and re-endothelialisation to BMS within three months post placement in the rabbit iliac artery. This is the first study to evaluate time-course changes of endothelial-dependent and endothelial-independent vasomotor function in combination with luminal surface coverage observation using SEM and en face immunohistochemistry measurement. Interestingly, despite indistinguishable stent surface endothelial cell coverage between groups, differential vasomotion was detected at 14 and 30 days, but resolving at 90 days. Therefore, we postulated that, for the newer generation of PES, in vivo arterial healing, which promotes restoration of physiological function, follows completion of drug/polymer release and thus lags morphologic endothelial recovery.
DEVELOPMENT OF THE NOVEL PES AND COMPARISON WITH TAXUS
Aside from the stent platform, the first-generation Food and Drug Administration (FDA)-approved polymer-based paclitaxel-eluting stent (TAXUS Express; Boston Scientific Corporation, Natick, MA, USA) contains two important components: a durable poly (styrene-b-isobutylene-b-styren) tri-block copolymer, which is a non-biodegradable synthetic molecule and is used to modulate optimal drug release over time, and the drug paclitaxel. Paclitaxel, the active component of the TAXUS stent, is a potent anti-restenosis compound which acts on cytoskeletal proteins by stabilising the microtubule β-tubulin subunit13. Higher paclitaxel doses have resulted in endothelial cell loss, fibrin deposition, medial necrosis and thrombus formation, reflective of a dose-dependent vascular injury pattern14. Data from published studies have shown that the permanent polymer can also induce local vascular hypersensitivity, inflammation and fibrin accumulation. At least partly due to polymer lipophilicity, only 10% of the initial paclitaxel dose is actually released from the commercial slow-release formulation. Therefore, the concomitant interactions of the residual retained drug and permanent polymer with the vessel wall may be instrumental in the pathogenesis of adverse events such as vessel wall healing delay, endothelial dysfunction, and stent thrombosis3-7,15-18. To avoid these undesirable side effects, this novel PES has been developed. Instead of 316L stainless steel platform, the newer generation of PES employed a PtCr alloy with high radiopacity. The decreased strut thickness (from 132 µm to 81 µm) and novel stent platform configuration have significantly enhanced the deliverability of the newer generation PES. Because the extent of vessel wall injury and subsequent smooth muscle cell (SMC) proliferation may be proportional to the stent strut thickness19, the benefit of decreasing strut thickness can also minimise SMC stimulation. The relative reduction of iron and nickel components in the current PES may mitigate the potential for allergy or hypersensitivity without sacrificing radial strength. Instead of a durable polymer coating, the newer generation PES utilises an abluminal biodegradable PLGA polymer. There is a 10-fold reduction in paclitaxel dose density from 1 µg/mm2 in the first-generation TAXUS Express stent to 0.1 µg/mm2.8 All of the above features are postulated to diminish vascular wall toxicity.
RE-ENDOTHELIALISATION AND VASOMOTOR FUNCTION
The endothelium is a monolayer organ with autocrine, paracrine and endocrine functions. Under healthy conditions, endothelial cells (EC) produce many vasoactive substances which maintain vascular homeostasis and normal vasomotor tone7. Therefore, evaluation of the completeness of endothelial regrowth is an essential component following endovascular device implantation. Both SEM and en face EC immunohistochemistry staining are standard in vitro analyses used to assess the luminal flow surface of EC morphology, as well as expression of functional proteins such as PECAM-1. In the current study, the stent tissue coverage was greater than 90% at 14 days, and no differences were found between groups for stent re-endothelialisation at any of the time-points. Analysis of the integrity of EC in vivo using physiologic and pharmacologic stimuli, such as the Ach vasomotor function test, can be used to serve as an important surrogate benchmark for arterial healing and functional recovery after DES implantation. Several clinical investigations have demonstrated that first-generation DES implantation was associated with long-term coronary endothelial dysfunction. Toni et al17 found a paradoxical vasoconstriction response in the peri-stent segments after DES placement at two to 12 months, while BMS responses were normal. Similarly, Shin et al18 and Kim et al20 reported that both TAXUS and Cypher (sirolimus-eluting) stents showed impairment of vasomotor function in response to Ach in segments distal to the stent at six to nine months. In concordance with the data of these patients, we also observed decreased vasorelaxation response to Ach at early time-points (14 and 30 days) in the rabbit iliac artery. Importantly, however, vasomotor function recovered fully by 90 days. Animal models play an instrumental role in the assessment of healing response to DES21. The stages of healing after rabbit interventional procedures follow a similar, but accelerated, pattern when compared with those of humans. However, any comparison of vessel wall response following DES in a healthy animal model with that in human atherosclerotic vasculature lesions is clearly inexact. The latter consist of lipid-rich plaque, with pre-existing inflammation, and a high potential for thrombogenesis. Therefore, implantation of DES in human lesions was shown to delay the healing process greatly3-7.
Study limitations
The relatively small number of study animals should be considered when interpreting these results. Moreover, thorough histopathology analysis, which was lacking in the present study, could help to address further the mechanisms of vasomotor dysfunction. According to published data, the chronic inflammatory reaction after DES implantation could induce reactive oxygen species activation, which leads to increasing cellular toxicity and impaired nitric oxide bioavailability and bioactivity. Inflammatory mediators may exert their effects on the distal vasculature both by diffusion and by vasa vasorum. Vasa vasorum interna originate directly from the arterial lumen and can extend over several centimetres along the arterial wall7,16,22. Therefore, the drug could be diffused directly to the downstream vessel wall via the micro-drainage channels.
Conclusions
Our findings demonstrate that a novel PES coated abluminally with bioabsorbable polymer, when compared to its identical BMS, results in comparable vasomotor function within three months post-implantation in a rabbit iliac model. Despite indistinguishable differences in endothelial cell coverage on the stent surface between groups, differential vasomotion was detected at early time-points, which may indicate that restoration of endothelial vasomotor function lags behind that of morphologic recovery following PES. Further work is required to evaluate the long-term safety and efficacy of this technology, as well as its clinical applicability.
Acknowledgements
The authors thank Bobby Price for his support with stent implant procedures. We also acknowledge the dedicated assistance of Irena Brants in managing the study execution.
Conflict of interest statement
D. Winsor-Hines, N. Sushkova, M. Eppihimer, B. Huibregtse, K.D. Dawkins, D. Hou are full-time employees of Boston Scientific. The other authors have no conflicts of interest to declare.

