
Abstract
Aims: Our aim was to evaluate arterial responses to paclitaxel and a novel fluorocopolymer-coated nitinol low-dose paclitaxel-eluting stent (FP-PES).
Methods and results: Human smooth muscle cell (SMC) migration was assessed after exposure to paclitaxel in vitro. For pharmacokinetics and vascular response, FP-PES or bare metal stents (BMS) were implanted in porcine iliofemoral arteries. Paclitaxel significantly inhibited human coronary and femoral artery SMC migration at doses as low as 1 pM. Inhibition was significantly greater for femoral compared with coronary artery SMCs from 1 pM to 1 μM. Pharmacokinetics showed consistent paclitaxel release from FP-PES over the study duration. The peak arterial wall paclitaxel level was 3.7 ng/mg at 10 days, with levels decreasing to 50% of peak at 60 days and 10% at 180 days. Paclitaxel was not detected in blood or remote organs. Arteriogram and histomorphometry analyses showed FP-PES significantly inhibits neointimal proliferation versus BMS at 30 and 90 days. Re-endothelialisation scores were not different between groups.
Conclusions: Paclitaxel affected femoral artery SMC migration at lower concentrations and to a greater degree than it did coronary artery SMCs. The novel FP-PES used in this preclinical study demonstrated a vascular healing response similar to BMS, while significantly inhibiting neointimal formation up to 90 days.
Abbreviations
ANOVA: analysis of variance
BMS: bare metal stent
DMSO: dimethyl sulphoxide
EEL: external elastic lamina
FP-PES: fluorocopolymer-coated paclitaxel-eluting stent
HCASMC: human coronary artery smooth muscle cell
HFASMC: human femoral artery smooth muscle cell
HPLC: high performance liquid chromatography
IEL: internal elastic lamina
PDGF: platelet-derived growth factor
PVDF-HFP: polyvinylidene fluoride-co-hexafluoropropylene
SEM: scanning electron microscopy
SMC: smooth muscle cell
Introduction
Endovascular interventions are commonly used to treat patients with peripheral vascular disease, particularly for the femoropopliteal artery1. However, durability of percutaneous therapy for peripheral artery disease remains challenging due to restenosis, which recent meta-analyses have suggested occurs in 40-70% of patients by one year2,3. Self-expanding nitinol bare metal stents (BMS) have demonstrated improved patency in some lesions, yet the BMS in-stent restenosis range is 20% to 40% at 12 months, and still higher in clinical practice2-5.
In the past decade, multiple randomised clinical trials have demonstrated reduced in-stent restenosis and target lesion revascularisation rates for drug-eluting stents compared with BMS in coronary artery disease6,7. A polymer-free paclitaxel-coated nitinol drug-eluting stent (Zilver® PTX®, 3 μg/mm2; Cook Medical, Bloomington, IN, USA) with a dose range from 220 to 880 µg showed favourable midterm and long-term outcomes for treatment of femoropopliteal in-stent restenosis8,9. However, delayed vascular healing and persistent inflammation were observed clinically by optical coherence tomography 12 months after Zilver PTX implantation10, and thrombus formation was reported at the uncovered stent strut area six months post implantation11.
Recently, a growing body of data has shown that polyvinylidene fluoride-co-hexafluoropropylene copolymer (PVDF-HFP)-coated material has excellent biocompatibility, thromboresistance, and rapid re-endothelialisation characteristics12-15. Therefore, a novel self-expanding nitinol stent with a low-dose paclitaxel-eluting coating including a fluorinated copolymer (FP-PES, 0.167 µg/mm2) with a dose range from 272 to 279 µg has been developed. The FP-PES development process included in vitro studies to characterise smooth muscle cell (SMC) responses to antiproliferative agents, and a porcine iliofemoral artery model was used to evaluate the pharmacokinetics and peripheral vascular biology response following FP-PES implantation.
Methods
IN VITRO STUDY: SMOOTH MUSCLE CELL TRANSMIGRATION
Detailed methods for the cell migration study of human coronary (HCASMC) and femoral (HFASMC) artery SMCs following exposure to paclitaxel are provided in the Online Appendix and summarised briefly here. HCASMCs or HFASMCs (Cell Applications, Inc., San Diego, CA, USA) were added to filter chambers prepared with transwell filter inserts along with paclitaxel dissolved in dimethyl sulphoxide (DMSO; vehicle control) to yield final concentrations ranging from 0.1 pM to 1 µM (n=4 per dose). Human platelet-derived growth factor (PDGF)-BB (100 ng/ml) was added as a chemoattractant. After incubating for 18 hours, SMCs that had migrated to the underside of the filter insert were stained with calcein-AM (5 µg/ml) and fluorescence was measured. Fluorescence data were normalised relative to the vehicle control of the respective cell type.
IN VIVO STUDIES: ANIMALS AND EXPERIMENTAL PROTOCOL
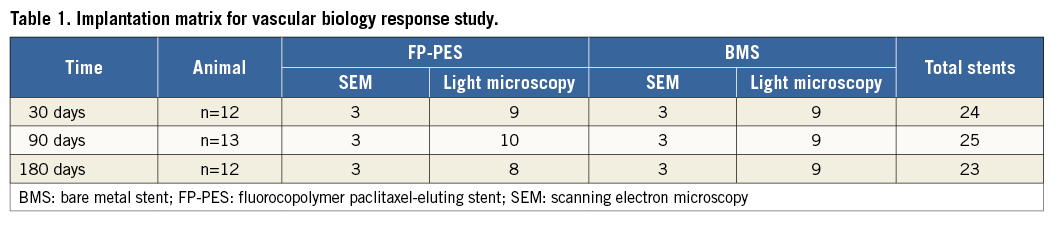
Animal handling and care followed the recommendations of the National Institutes of Health guide for the care and use of laboratory animals. All protocols were approved by the animal care and use committee of our research institute and followed the Association for Assessment and Accreditation of Laboratory Animal Care guidelines. A total of 49 juvenile Yorkshire farm pigs (30-40 kg) were used in the pharmacokinetics (n=12 pigs) and vascular biologic response (n=37 pigs) studies. The implantation matrix of the vascular response study is listed in Table 1. All animals received a combination of 81 mg aspirin and 75 mg clopidogrel by mouth daily for three days prior to stent implantation until termination and fasted overnight before the procedure. The animals were sedated by intramuscular injection of tiletamine and zolazepam (4 mg/kg) and xylazine (2 mg/kg). After intubation, general anaesthesia was induced and maintained with isofluorane (2.5%).
The endovascular intervention was performed at a digital X-ray laboratory through carotid cut-down following heparinisation (200 U/kg). Stents oversized 1.0 to 2.0 mm to the baseline vessel diameter were implanted bilaterally in iliofemoral arteries according to a constrained randomisation scheme.
Repeat arteriography was performed only for the vascular response study animals before termination at 30, 90 and 180 days post implantation. Quantitative arteriography was performed by an independent observer, blinded to the study assignment. From non-superimposed and non-foreshortened views, reference diameters were taken from proximal and distal portions of the vessel at the treated sites and the stent-to-artery ratio was calculated. Minimum luminal diameter within the stented segments was also measured. The in-stent late lumen loss was calculated as the difference between minimum luminal diameter at follow-up and at post stent baseline. Prior to arteriogram, nitroglycerine (200 µg) was administered by bolus injection.
STENT CHARACTERISTICS
The FP-PES (Eluvia™ Drug-Eluting Vascular Stent System; Boston Scientific, Marlborough, MA, USA) is a self-expanding nitinol stent with radiopaque tantalum markers at each end. It is coated with a conformal coating of a poly-n-butyl methacrylate primer layer followed by a PVDF-HFP layer of the active pharmaceutical ingredient paclitaxel with a dose density of 0.167 µg/mm2. The test stents were 6.0, 7.0, or 8.0 mm in diameter and 20 mm (pharmacokinetic study) or 80 mm (vascular response study) in length. The total amount of paclitaxel coated on the stent ranged from 272 to 279 µg based upon stent diameter. All devices were packaged and sterilised individually before use. An FDA-approved self-expanding nitinol stent (Epic™; Boston Scientific) was used as a control.
PHARMACOKINETICS STUDY
Twelve pigs were included for in vivo pharmacokinetic analysis at four, 10, 30, 60, 90 and 180 days (eight stents per time point). Each animal received two non-overlapping FP-PES bilaterally in the iliofemoral arteries. Stent drug content and drug levels at the local artery wall, non-stented proximal and distal arteries, and downstream supplying muscle, as well as systemic blood levels were measured. After termination, the stented vessels along with 15 mm of proximal and distal reference arteries were flushed and excised from the animal. After removing the immediately adjacent proximal and distal reference arteries, the stent and its surrounding tissue were carefully separated under a stereoscopic microscope. Caution was used to minimise contact between the surgical instruments and the stent to reduce the potential of damaging the drug coating. Finally, the isolated stent, its surrounding artery, and proximal and distal reference arteries were placed in separate vials and frozen at –80°C.
Blood, downstream skeletal muscle, heart, lung, liver, spleen, and kidney were also collected and frozen for drug content assessment. All tissue samples were analysed for paclitaxel content using high performance liquid chromatography (HPLC). The residual paclitaxel on the stent was also assessed.
VASCULAR RESPONSE STUDY
Thirty-seven pigs were randomly assigned to bilateral implantation with either FP-PES or BMS in the iliofemoral arteries. Eighteen stented arteries were assessed for endothelial coverage by scanning electron microscopy (SEM), and fifty-four stented arteries were evaluated for vascular response by light microscopy at 30, 90 and 180 days. At explant, the stented vessels were perfusion-fixed with 10% neutral buffered formalin. Implanted arteries were bisected longitudinally and processed for SEM as previously described16. Due to the length of the stented segment (80 mm), SEM images of luminal surface and endothelial cells were acquired at the proximal, middle, and distal stented regions. Re-endothelialisation was semi-quantitatively graded as 0 (>90% of the luminal circumference), 1 (75-90%), or 2 (<75%).
The stented segments used for histopathology and histomorphometry analysis were plastic-embedded. Sections from the proximal, middle, and distal stented regions were cut with a heavy-duty microtome equipped with a tungsten-carbide knife. All cross-sections were stained with haematoxylin-eosin, and semi-quantitatively scored for both internal and external elastic lamina (IEL/EEL, 0=no or minimal disruption, 1=≤25% disruption, 2=25 to 50% disruption, 3=50 to 75% disruption, 4=≥75% disruption), luminal thrombus, re-endothelialisation (0=≥90%, 1=75-90%, 2=≤75% of the luminal circumference coverage), inflammation as well as fibrin deposition (0=not present, 1=mild, 2=moderate, 3=severe). Morphometric analysis was performed by computerised planimetry on proximal, mid, and distal sections cut from each stented vessel. The neointimal area was obtained by subtracting the lumen area from the internal elastic lamina area. The intimal thickness at each stent strut site was also measured. The histologic percent area stenosis (%AS; [1–luminal area/neointimal area]×100) was calculated.
STATISTICAL ANALYSIS
Histomorphometric and SMC migration data were determined to be normally distributed with equal variances using the Shapiro-Wilk and Levene tests, respectively. SMC migration was analysed with one-way ANOVA with multiple comparisons using the Bonferroni method. Comparisons between HCASMCs and HFASMCs at each dose were performed by t-test. Histomorphometric data were compared between BMS and FP-PES at each time point by t-test. Morphologic data (ordinal values) were compared between BMS and FP-PES at each time point by Mann-Whitney test. A p-value <0.05 was considered significant.
Results
SMOOTH MUSCLE CELL MIGRATION
Paclitaxel inhibited HFASMC and HCASMC migration to PDGF-BB at doses as low as 1 pM: approximately ~80% less for HFASMCs vs. 40% less for HCASMCs relative to vehicle control (Figure 1). Greater inhibition of SMC migration occurred in both cell types with increasing paclitaxel concentration (p<0.0001) (Figure 1). In HFASMC, exposure to increasing paclitaxel concentrations resulted in a gradual and significant decrease in migration to ~16% of control at 1 nM (p<0.05) with no significant additional inhibition at concentrations up to 1 μM. In HCASMC, migration significantly decreased to ~12% of control at 100 nM (p<0.05), with no additional inhibition at 1 μM (Figure 1). HFASMC migration was significantly reduced compared with HCASMC migration at paclitaxel concentrations ≥1 pM (p<0.05).
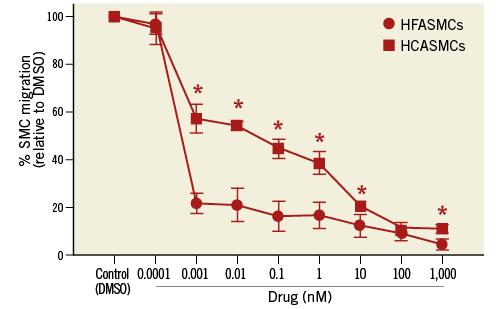
Figure 1. SMC migration. HCASMC and HFASMC migration in the presence of paclitaxel, relative to DMSO control. *p<0.05 HCASMC vs. HFASMC.
ARTERIOGRAPHY CHARACTERISTICS AND STENT INTEGRITY
All animals tolerated the stent deployment procedure without complications, and no physical activity limitations or lower extremity injury were observed during follow-up. There were no differences with regard to baseline target vessel diameter between FP-PES and BMS. All vessels were angiographically patent at repeat exam. The stent-to-artery ratio was higher for FP-PES vs. BMS at both 30 days (mean 1.17±0.07 vs. 1.06±0.13) and 90 days (1.23±0.11 vs. 1.06±0.08; p<0.05, respectively). In-stent lumen loss was significantly reduced in FP-PES compared with BMS at 30 days (-0.47±0.39 vs. 0.64±0.63 mm, p<0.001), and the difference was no longer measurable from arteriogram at later time points.
PHARMACOKINETICS IN VIVO
Paclitaxel release from the FP-PES was steady with no burst phase (Figure 2A). About 27% of the total loading dose was released from the stent by day four and 73% by day 180 (Figure 2A). Accordingly, the mean drug concentration measured in the arterial wall ranged from 3.72 ng/mg at 10 days to 0.38 ng/mg at 180 days (Figure 2B). In the proximal and distal arterial tissue, paclitaxel was only detected at days four and 10, and was typically lower than in stented artery segments by 100-fold (proximal) and 10-fold (distal). Major organ, downstream skeletal muscle, and systemic paclitaxel levels were below the HPLC lower limit of quantification at all time points.
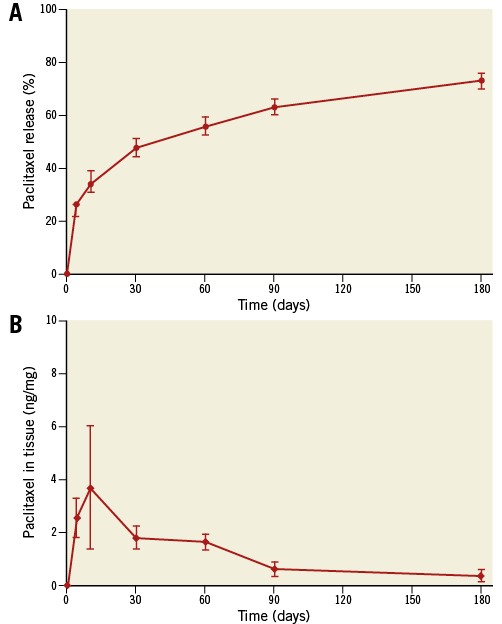
Figure 2. Paclitaxel pharmacokinetics in vivo. A) In vivo release of paclitaxel based on stent content. Paclitaxel was released steadily with a limited initial burst phase followed by a sustained controlled release. Seventy-three percent of total loading dose was released in vivo at 180 days. B) Paclitaxel in arterial tissue. The peak tissue level in the arterial wall was 3.7 ng/mg at 10 days, and slowly decreased to 10% of peak at 180 days.
VASCULAR BIOLOGY RESPONSE ANALYSES
The IEL and EEL disruption scores were not different between groups at each time point (Online Figure 1). Both FP-PES and BMS groups had thin neointimal formation. No thrombi adhesion was detected on the luminal surfaces by either gross or microscopic examination at any time point. SEM observation revealed a layer of flattened endothelial or endothelial-like cells covering the stent struts. The covered stent struts were visible and easily traced at both 30 and 90 days, especially for FP-PES-stented vessels. However, stent strut visibility was diminished at 180 days in both groups (Figure 3). The overall degree of re-endothelialisation (above and between stent struts) detected by SEM did not differ between FP-PES and BMS at any of the three time points (Online Table 1).
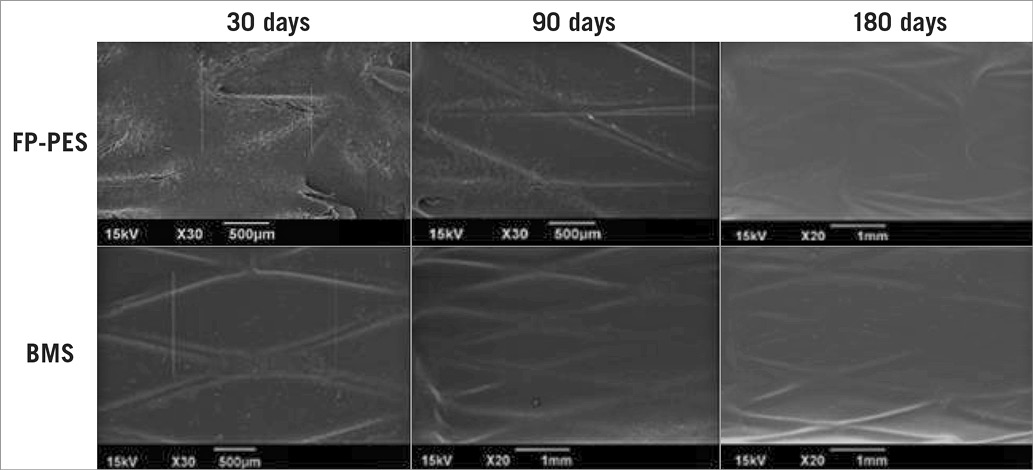
Figure 3. Scanning electron microscopy shows comparable coverage of luminal surface for FP-PES and BMS. The stent strut was readily visible at 30 and 90 days, especially FP-PES specimens. However, the visibility was diminished along the time course. No intramural thrombus was observed.
As with the SEM observations, stented arteries in both groups displayed equivalent re-endothelialisation scores at all time points in histopathologic analysis with light microscopy (Table 2). However, a drug effect was apparent in the form of more substantial peri-strut fibrin deposits as well as an inflammation response for the paclitaxel-eluting stent compared to BMS. FP-PES had higher average fibrin deposition scores at all three time points (p<0.001) (Table 2). Importantly, the fibrin grade for FP-PES groups reduced significantly over time, with the lowest scores at 180 days (p<0.001 vs. both 30 days and 90 days). Although the absolute inflammation score was low in both groups, FP-PES had higher scores at 30 and 180 days (Table 2).
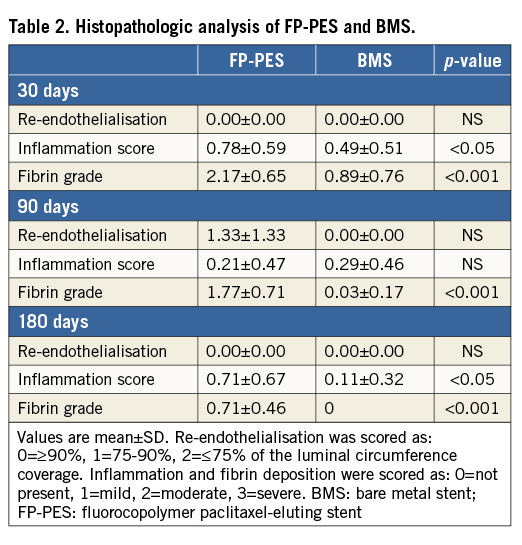
With respect to morphometric measurement under light microscopy, the neointima formation was clearly suppressed by FP-PES compared with BMS at both 30 and 90 days (Table 3), yielding 36.4% less in-stent area stenosis for FP-PES versus BMS at 30 days (p<0.001) and 33.6% less at 90 days (p<0.001). Differences between groups were not observed at 180 days. Low-magnification light microscopy images in Figure 4 illustrate overall vessel wall morphologies at 30 days, 90 days and 180 days (Online Figure 2).
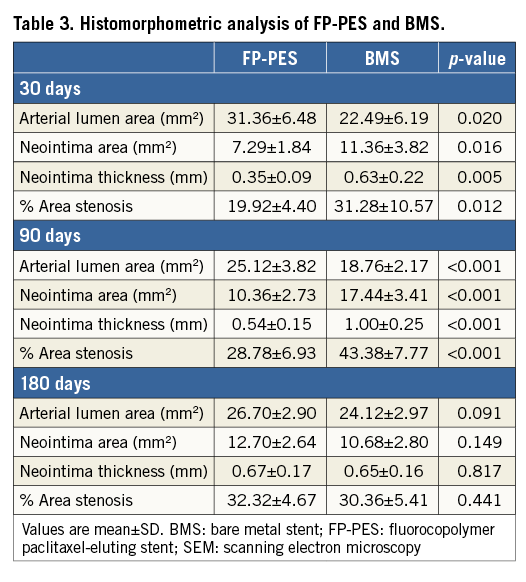

Figure 4. Low magnification (2.5×) light microscopic images of haematoxylin-eosin stained sections. This demonstrates overall vessel morphologies. Suppression of neointimal formation was observed in FP-PES versus BMS at both 30 and 90 days.
Discussion
The study provides a comprehensive evaluation of the pharmacokinetics and long-term vascular response to a novel fluorocopolymer-coated low-dose paclitaxel-eluting stent in a porcine iliofemoral model. Pharmacokinetic analysis of the stented vessels showed a measurable paclitaxel level in the arterial wall until 180 days, with none detected in plasma. Neointimal formation associated with the FP-PES was less than with BMS at 30 and 90 days in this porcine iliofemoral artery model. This inhibition of neointimal formation was accompanied by similar healing responses between FP-PES and BMS, suggesting that the paclitaxel levels released by FP-PES into the vessel wall did not negatively impact on re-endothelialisation. This pattern is reminiscent of the initial work with human coronary artery cells by Axel et al17, in which lower paclitaxel doses sufficient to inhibit HCASMC migration did not inhibit HCA endothelial cell growth. The current in vitro study demonstrates that paclitaxel suppresses HFASMC migration to a greater degree than HCASMC migration across a broad dose range, suggesting that paclitaxel may inhibit neointima formation in the femoral artery at lower doses than would be needed for coronary arteries. Therefore, while the paclitaxel concentrations were within the range for inhibition of coronary SMC proliferation (56 nmol/L to 50 µmol/L)17 at the early time points, the levels at the later time points may be sufficient for peripheral cellular response.
Although low-dose paclitaxel did not inhibit HCA endothelial cell growth, Axel et al demonstrated that high-dose paclitaxel was a potent inhibitor of both coronary artery SMC and coronary artery endothelial cell proliferation and migration17. Correspondingly, a dose-dependent decrease in neointimal formation and subsequent increase in vessel wall toxicity resulting in endothelial cell loss, severe fibrin deposition, and thrombus formation was reported in preclinical studies18-20. Therefore, careful selection of the ideal dose and release kinetics to maximise therapeutic effect in the femoral artery while minimising vessel wall toxicity should be essential factors when coating paclitaxel on a peripheral stent21. The in vitro study results suggest that a low dose of paclitaxel may be sufficient to reduce SMC migration in the femoral artery, providing some justification for selecting a low dose for the in vivo study. Furthermore, the FP-PES elutes paclitaxel over an extended period of time. Compared to that reported for Zilver PTX (3 μg/mm2), there is an 18-fold reduction in paclitaxel dose density in FP-PES (0.167 μg/mm2). Indeed, this novel low-dose FP-PES not only exhibited the therapeutic goal, but also minimised vascular wall toxicity. In addition, the twelve-month results of the MAJESTIC trial showed that the FP-PES had a 96.1% primary patency without stent fractures, deaths or amputations22.
PVDF-HFP fluorinated copolymer is a semi-crystalline material with saturated carbon-carbon single bonds. The backbone carbon atoms are more than 50% fluorinated, conferring a high degree of chemical inertness due to C-F bond dissociation energy, and contributing to the polymer’s hydrophobic properties. Due to the absence of reactive or enzymatically sensitive components, the polymer is resistant to hydrolytic, oxidative, or enzymatic cleavage. Consistent with the present preclinical findings, the fluorocopolymer coating has demonstrated biocompatibility and haemocompatibility, and is benign to re-endothelialisation12-15. A meta-analysis of 13 randomised trials involving 17,097 patients further confirmed that use of a fluorocopolymer-coated everolimus-eluting stent in coronary arteries was associated with highly significant reductions in stent thrombosis, with concordant reductions in target vessel revascularisation and myocardial infarction compared with non-fluorocopolymer-coated stents23. These results may further support the safety of the fluorocopolymer in peripheral vascular applications.
It is well known that the degree of vascular injury correlates with in-stent neointimal proliferation24. Although the stent-to-artery ratio in the FP-PES group compared with the BMS group from the current study was significantly higher at 30 and 90 days, the inhibition effect on neointimal proliferation was clearly shown both angiographically and histomorphometrically (Table 3). At 180 days, complete healing with a response similar to BMS further demonstrated the safety of the FP-PES. Overall, the current study demonstrated that the novel FP-PES showed a balance between therapeutic neointima suppression and vascular healing.
Limitations
The relatively small number of animal subjects should be considered and the statistical analysis interpreted accordingly. Biologic results in normal porcine iliofemoral arteries after FP-PES implantation may not be representative of those in diseased human peripheral artery lesions, which consist of atherosclerotic and potentially thrombotic, severely inflammatory, and calcified stenotic lesions2-5. The porcine iliac artery model has limitations in terms of length and biomechanics in terms of translation to the human superficial femoral artery. Although most of the drug was released at 180 days, the drug elution kinetics were still incomplete, and longer-term studies are needed to confirm the total drug released in vivo.
Conclusions
Maintaining long-term patency after peripheral endovascular intervention remains a challenge. Our findings demonstrated that, compared with BMS, FP-PES inhibited neointimal formation at 30 days and 90 days, but had a vascular healing response similar to the bare metal stent.
| Impact on daily practice Pharmacokinetics and the vascular response to a fluorocopolymer-coated low-dose paclitaxel-eluting stent implanted in a preclinical porcine iliofemoral model were investigated. Paclitaxel was released gradually and was present in arterial tissue up to 180 days. Angiographic and histologic analyses showed neointimal inhibition with the drug-eluting stent vs. bare metal, with similar vascular healing between groups. These preclinical findings suggest a sustained antiproliferative effect of the fluoro-copolymer-paclitaxel coating in peripheral arteries, without adverse effects on healing. |
Acknowledgements
The authors express gratitude to Elizabeth Davis, PhD (Boston Scientific, Maple Grove, MN, USA) for editorial assistance.
Funding
This study was supported by a research grant from Boston Scientific.
Conflict of interest statement
All the authors are employees of and hold stock in Boston Scientific Corporation.
SUPPLEMENTARY DATA
Online Appendix. Supplemental methods
IN VITRO STUDY: SMOOTH MUSCLE CELL TRANSMIGRATION
Human coronary and femoral artery smooth muscle cells (HCASMC and HFASMC, respectively; Cell Applications, Inc., San Diego, CA, USA) were grown in tissue culture flasks at 37ºC and 5% CO2 with SMC growth medium (Cell Applications, Inc.) specified by the manufacturer. All experiments were performed on cells between passages four and six.
Flasks of near confluent HCASMCs and HFASMCs were cultured in SMC basal media with 0.5% foetal bovine serum for 24 hours. Transwell filter inserts (BD FluoroBlok, 8 mm pore diameter; Becton Dickinson, Franklin Lakes, NJ, USA) were prepared in 96-well plates by treating the surface of the filter membrane with 5 µg/ml of human fibronectin (Sigma-Aldrich Corp., St. Louis, MO, USA), dissolved in sterile distilled water, at 4ºC for 16 hours. The fibronectin solution was removed and the filter allowed to air dry for one hour. Flasks of SMCs were rinsed in phosphate-buffered saline (PBS; Life Technologies, Beverly, MA, USA) and treated with 0.25% Trypsin-EDTA (Life Technologies). SMC suspensions were washed by centrifugation and re-suspended in SMC basal media with 0.5% foetal bovine serum. HCASMCs or HFASMCs (5,000 cells per transwell) were added to the top (apical) side of the filter chamber. Paclitaxel (Sigma-Aldrich Corp.) dissolved in dimethyl sulphoxide (DMSO) (vehicle control; Sigma-Aldrich Corp.) was added to the cell suspension to yield final concentrations ranging from 0.1 pM to 1 µM (n=4 per dose). Human platelet-derived growth factor (PDGF)-BB (R&D Systems, Inc., Minneapolis, MN, USA) in SMC basal media with 0.5% foetal bovine serum was added to the bottom of the 96-well plates at a concentration of 100 ng/ml to serve as a chemoattractant. Plates containing transwell filters were placed in an incubator at 37°C and 5% CO2 for 18 hours. The transwell insert was then removed and placed in a new plate with wells containing calcein-AM (5 µg/ml; Sigma-Aldrich Corp.) for 90 minutes at 37ºC to stain SMCs that had migrated to the underside of the filter insert. Fluorescence on the bottom side of the filter was measured at excitation/emission wavelengths of 485/530 nm using a fluorescent bottom-reading plate reader (SpectraMax® M5; Molecular Devices, Sunnyvale, CA, USA) and is expressed in relative fluorescent units (RFU). Data were normalised relative to the RFU for the vehicle control of the respective cell type.
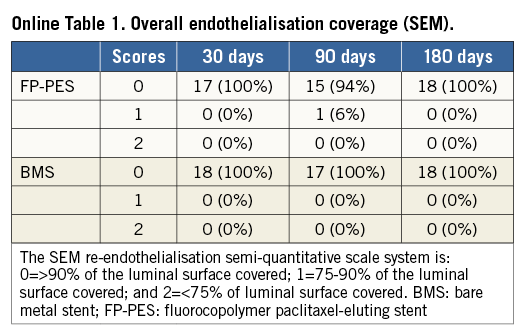
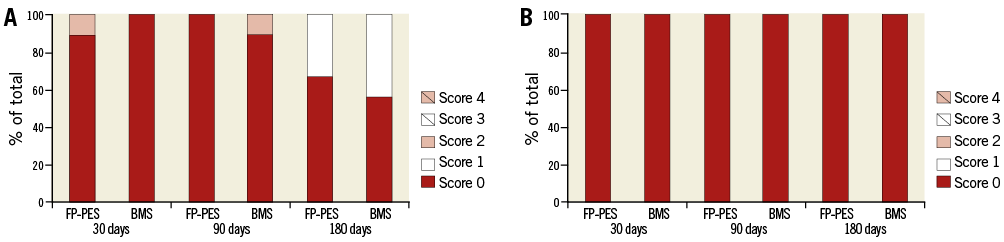
Online Figure 1. Disruption of internal and external elastic lamina. A) Internal elastic lamina (IEL) disruption scores. B) External elastic lamina (EEL) disruption scores. Score 0=no or minimal disruption, 1=≤25% disruption, 2=25 to 50% disruption, 3=≥50 to 75% disruption, 4=≥75% disruption.
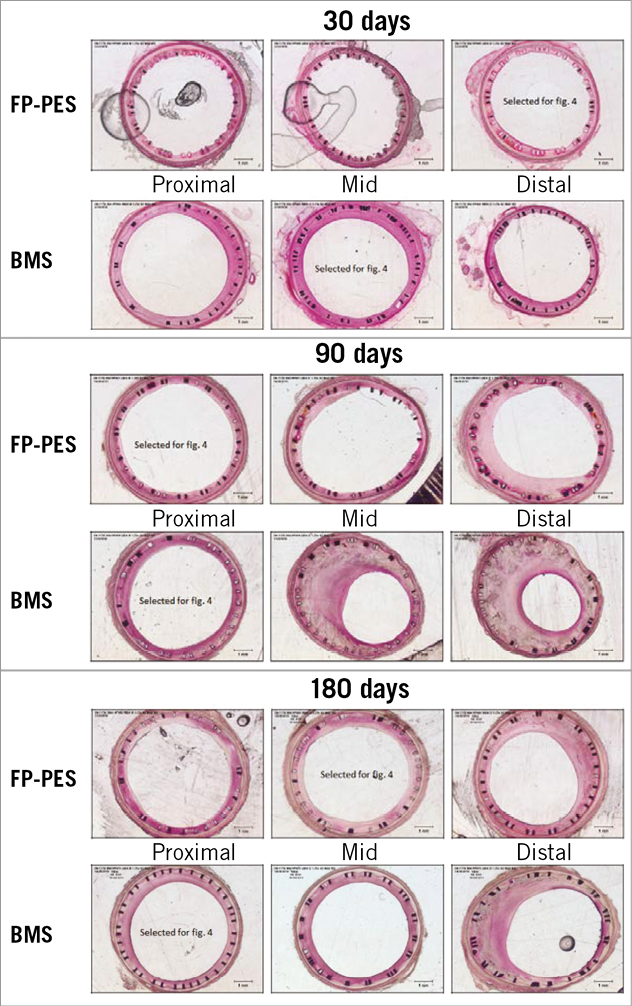
Online Figure 2. Low magnification (2.5×) light microscopic images of haematoxylin-eosin stained sections. Full array of stent segment images corresponding with Figure 4 in the main text. Shows inhibition of neointimal formation at both 30 and 90 days. Fibrin deposition was seen and diminished along the time course.

