- restenosis
- paclitaxel
- local drug delivery
- paclitaxel-coated balloon catheter
Abstract
Our initial investigations into restenosis inhibition by local drug delivery were prompted by reports on an improved outcome of coronary interventions, including a lower rate of target lesion revascularisation, when the intervention was performed with an ionic instead of non-ionic contrast medium. Although this was not confirmed in an animal study, the short exposure of the vessel wall to paclitaxel dissolved in contrast agent or coated on balloons proved to be efficacious. A study comparing three methods of local drug delivery to the coronary artery in pigs indicated the following order of efficacy in inhibiting neointimal proliferation: paclitaxel-coated balloons > sirolimus-eluting stents, sustained drug release > paclitaxel in contrast medium. Cell culture experiments confirmed that cell proliferation can be inhibited by very short exposure to the drug. Shorter exposure times require higher drug concentrations. Effective paclitaxel concentrations in porcine arteries are achieved when the drug is dissolved in contrast medium or coated on balloons. Paclitaxel is an exceptional drug in that it stays in the treated tissue for a long time. This may explain the long-lasting efficacy of paclitaxel-coated balloons, but does not disprove the hypothesis that the agent blocks a process initiating long-lasting excessive neointimal proliferation, which occurs early after vessel injury.
Introduction
With the advent of angioplasty restenosis of successfully opened vessel became an issue. The frequency of restenosis has been somewhat diminished by the introduction of stents; however, the patho-histology of the lesions changed, and the medium- and long-term success rate of the treatment of dilated and re-narrowed lesions decreased. Attempts have been made to prevent restenosis by new mechanical devices, systemic pharmacotherapy, percutaneous radiation therapy, brachytherapy and a variety of methods for image-guided local drug administration, all of them without convincing results1,2. Many of these methods make treatment much more complicated and some of them also more invasive. After the first reports on the success of clinical trials using drug-eluting stents, efforts have been focused on targeted drug therapy by slow release formulations coated on the stent struts, a method which provides long-lasting local drug concentrations in the diseased tissue with minimal systemic exposure and risk of adverse effects to distant organs. Furthermore, the slow development of restenosis and lack of efficacy of medium and fast release formulations on stents suggested that slow release is the only successful approach to restenosis inhibition3,4. This opinion was further supported by the initial failure of various methods by which dissolved drugs were injected into the vessel lumen or vessel wall5.
Contrast media for restenosis inhibition?
We entered this field at the time of the early enthusiasm for drug-eluting stents through a side door. At that time Dr. Speck came across a paper by Batchelor et al in the American Journal of Cardiology6 reporting on a tendency towards a reduced incidence of various adverse cardiac events up to 30 days following the use of an ionic contrast medium (Hexabrix™, sodium meglumine ioxaglate) in PTCA for acute myocardial infarction compared to non-ionic contrast media. This was explained by the known anticoagulant effect of the ionic contrast medium. Shortly afterwards, Bruno Scheller and colleagues7 reported a statistically significant decrease in the incidence of target lesion revascularisation and the combined clinical endpoint of coronary artery bypass grafting, target lesion revascularisation and overall death within 12 months following the use of a ionic versus a non-ionic contrast agent during PTCA and stent implantation. There were a variety of potential explanations, e.g., (a) although the study included about 4,000 patients it was a not randomised, therefore, doubts in the results were justified; (b) inhibition of blood clotting or microthrombi apposition on the injured vessel wall by the ionic contrast agent and, therefore, less mitogenic stimulus; (c) a direct toxic effect of the ionic contrast agent on the vessel wall reducing the capability to proliferate. Acontinuous pharmacological effect of the contrast agents due to prolonged presence in the vessel or vessel wall could be ruled out because it was known that the common X-ray contrast media do not enter cells and are rapidly eliminated from the extracellular space. Occasionally, short-lasting contrast was, however, visible adjacent to the vessel wall even after the lumen was cleared. This observation pointed to a somewhat longer persistence of the contrast medium on the surface of the vessel wall, but could hardly explain the long-lasting effect observed in the patients.
After discussing the findings of the trial, the potential mechanisms of action of the ionic contrast medium, and options to further investigate the phenomenon, while also considering the long duration and expense of a randomised clinical trial, Bruno Scheller proposed a study in pigs. In this study, an ionic and a non-ionic contrast medium could be compared with regard to neointimal proliferation stimulated in the coronaries by overstretching and stent implantation. To make the study more informative we added a third type of contrast agent, a non-ionic isotonic dimer (similar to Visipaque™, iodixanol), and considered mixing an antiproliferative agent to one of the contrast media. The rationale was that any beneficial effect of a contrast medium could be copied or enhanced by a more powerful drug. In any case, contrast media have to be selectively injected in the coronary arteries, are usually well tolerated, and allow the observation of the distribution of the drug.
Paclitaxel was selected as a suitable drug because of its proven efficacy on stents, the approval for systemic tumour therapy8, and the availability of the material manufactured according to the guidelines for pharmaceutical products (GMP, good manufacturing practice). Afirst problem to overcome was the extremely low water solubility of paclitaxel. We expected that it would be almost impossible to achieve therapeutically useful concentrations in the aqueous contrast media. To our great surprise, it was possible to reach much higher concentrations of paclitaxel in contrast media than in saline. Nevertheless, in the first study in pigs, we used a paclitaxel derivative with a somewhat better water solubility, namely protaxel9.
The study comprised four treatment groups, four animals/group, two coronary arteries treated per animal with 20-30% overstretch and stent implantation:
(a) the ionic contrast medium Hexabrix™, 320 mg iodine/ml; 580mosm
(b) the non-ionic monomeric Ultravist™, 370 mg iodine/ml; 770mosm
(c) Iosimenol, a non-ionic isotonic dimeric contrast agent, 350mg iodine/ml, 300 mosm10
(d) Ultravist™-370 plus 74µm protaxel.
Re-angiography and histomorphometry four weeks after treatment indicated similar in-stent stenosis due to neointimal proliferation in the groups treated with the contrast media belonging to three different chemical classes11, but a statistically significant reduction of late lumen loss, neointimal area and related measures of lumen narrowing in the group treated with protaxel in the contrast medium12 (Figure 1). In conclusion, the clinical findings favouring the use of ionic contrast media were not reproduced, but a reduction of neointimal proliferation by the taxane was observed, in spite of the short contact time of the freely flowing solution during opacification of the vessel.
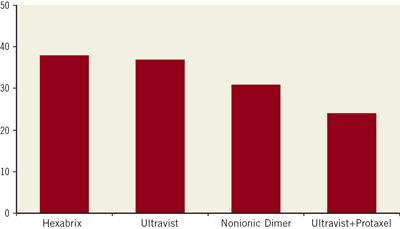
Figure 1. Impact of intracoronary administration of various contrast media preparations on neointimal proliferation following stent implantation in coronary arteries of pigs. % diameter stenosis; Ultravist vs. Ultravist + Protaxel: p<0.05.
Paclitaxel in contrast media
The contrast medium as carrier for a drug is also appealing because the whole vessel with several lesions is treated simultaneously. First, the above-reported result had to be reproduced in a second experiment. Since protaxel was neither an approved drug nor commercially available, we switched to paclitaxel and improved the formulation, allowing drug concentrations of up to 200µm. The same animal model and study design was used. Four groups of animals were treated in the following way:
(a) 80 ml contrast medium without additives for the visualisation of the coronary arteries
(b) same as (a) but additionally 80 ml contrast medium + 200µm paclitaxel intravenously
(c) 80 ml contrast medium with 100µm paclitaxel for the visualisation of the coronary arteries
(d) 80 ml contrast medium with 200µm paclitaxel for the visualisation of the coronary arteries
Re-angiography and histomorphometry after four weeks confirmed the inhibition of neointimal proliferation by the drug when directly injected into the coronary arteries. Systemic (intravenous) administration was completely inefficacious (Figure 2). In spite of the injection directly into the coronary arteries, the drug was tolerated without any effect on the ECG, acute cardiac function or contractility after four weeks. Histology did not reveal any signs of toxicity in the treated vessels or the myocardium13.
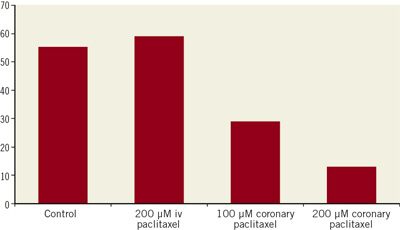
Figure 2. Impact of intracoronary or intravenous administration of various contrast media preparations on neointimal proliferation following stent implantation in coronary arteries of pigs. % diameter stenosis; control (no paclitaxel) vs. 200µm paclitaxel: p<0.01.
The decisive question was whether the favourable findings in animals could be reproduced in patients. To this end a phaseI clinical trial was initiated in patients with de novo stenosis in acoronary artery to be treated by stent implantation aimed at investigating the tolerance of paclitaxel dissolved in Ultravist™-370. First, the very low concentration of 10µm paclitaxel in the contrast agent was administered. In three steps the paclitaxel concentration was increased to the 200 µm tested in the animal study, each step depending on the unequivocal tolerance of the previous dose level. At each dose level, eight patients were treated using two different preparations in a randomised and blinded way, two patients with Ultravist™ without the drug and six patients with Ultravist™ with the drug. All preparations and dose levels were equally well tolerated. The number of patients was far too low to rule out rare adverse events or to test efficacy with regard to restenosis inhibition. Nevertheless, re-angiography after six months indicated a tendency in favour of restenosis inhibition by the drug-containing contrast medium in terms of late lumen loss, binary restenosis rate, and target lesion revascularisation14 (Table1).

Paclitaxel on balloon catheters
From the studies with paclitaxel dissolved in the contrast agent, it was concluded that in spite of the short contact time to the vessel wall, neointimal proliferation was inhibited for at least four weeks, the duration of the experiments. If such a short contact time should be confirmed to be sufficient, then angioplasty balloons could be considered as a carrier. The coating of smooth regular PTCA balloons with a drug is not trivial; requirements include the right dose, sufficient adhesion for handling before and on the way to the lesion, fast release of the drug, fast transfer into the vessel wall, efficacy and safety. One of the surprising findings during the initial experiments was that the balloons could be coated in the folded state, thus avoiding the loss of drug during folding. This was extremely important as we had no access to expensive equipment, which is required to properly fold the balloons. Although the distribution of the drug on the balloon was circumferentially not uniform, this did not impair its distribution on the luminal surface of the vessel wall nor its efficacy15. In a first animal study, the dose and an effective formulation containing a small proportion of the X-ray contrast medium Ultravist as a dry matrix for paclitaxel were defined16.
It was obvious that local paclitaxel delivery to the arterial wall was possible with three different carriers: the stent, the solution, and the balloon. At that time the Cypher™ stent was already commercially available and obviously the gold standard with which new methods had to be compared. We tested all three methods in the porcine coronary model: the drug-eluting stent, the drug on the balloon, and the drug in the contrast medium solution. The result confirmed inhibition of neointimal proliferation by all three methods; however, the coated balloon and the drug-eluting stent were more efficacious than the drug dissolved in contrast medium.
Performance in patients
The studies in the animal model could not answer the question whether the new methods, in spite of the short duration of the exposure of tissue to the drug, would provide long-term protection from restenosis in the same way as drug-eluting stents do. Healthy arteries of young pigs are very different from severely atherosclerotic vessels in elderly patients. Furthermore, the duration of action cannot be tested in the pig model because even sustained release from drug-eluting stents suppresses neointimal proliferation only for about one month17.
Several randomised clinical trials were initiated only shortly after the results of the studies in animals became available. They confirmed the efficacy of the treatment by short-lasting contact of the vessel wall to the drug-coated balloon18-21. Meanwhile, 2- and 5-year follow-up of the patients indicate that the benefit of the treatment persists over time.
One of these initial clinical trials shall be mentioned because it provides information that may help to better understand the predictive value of the animal model. Since the treatment of a larger vessel segment could prove advantageous compared to the strictly limited area covered by drug-eluting stents, and the only slightly larger area treated by drug-coated balloons, Tepe and co-workers decided to perform a randomised head-to-head clinical trial of paclitaxel in contrast medium and paclitaxel-coated balloons versus plain balloon angioplasty in superficial femoral and popliteal arteries. Six-month late lumen loss in the treated lesion was the primary endpoint. The results confirmed the inhibition of restenosis by the drug-coated balloons but no effect of paclitaxel dissolved in the contrast medium, except for a slight tendency toward a reduced number of target-lesion revascularisations20. The failure may be explained by the weaker effect of the liquid preparation in the animal model and by the larger diameter of the femoral arteries, which favour flow in the centre of the lumen rather than vessel wall contact.
How can long-lasting inhibition of neointimal proliferation after a single short contact to the drug be explained?
Neointimal proliferation following angioplasty is triggered by vessel injury and subsequent events such as platelet deposition, inflammatory reactions and the secretion of endogenous mitogens. Inflammation may either be self-limiting or develop into a self-perpetuating process. Once successfully interrupted, this process may not spontaneously recur. We assumed that short exposure to a sufficiently high concentration of a suitable drug would prevent a process right from the start which otherwise stimulates neointimal proliferation or would interrupt the early chain of events resulting in continuous excessive cell proliferation.
The growing knowledge of the very exceptional pharmacokinetic properties of paclitaxel, however, points to an alternative explanation for the persistent effects of a single, immediately released dose of this drug.
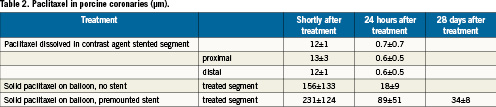
Effective concentrations of paclitaxel inhibits proliferation of cells in vitro for several days to two weeks depending strongly on exposure times. Axel et al22 reported almost complete inhibition when cells were exposed for 20min to 1µm paclitaxel, whereas an about 100-fold lower concentration was sufficient for continuous exposure to the drug. Depending on the absence or presence of the contrast medium iopromide, Clever et al23 found 50 to 75% inhibition of cell density after only 3-second exposure at 15µm paclitaxel concentration. A comparison with in vivo data indicates that, at least early on, these concentrations are reached or surpassed in the vessel wall (Table 2). Whereas drug-eluting stents continuously supply the drugs, the administration with the contrast medium or balloon as carrier deposits a limited amount of the drug in the tissue. Initial concentrations of paclitaxel in the vessel wall after angiography with paclitaxel dissolved in the contrast medium reach about 1-10µm, decreasing to <1µm already after 24hours. This is close to the minimum concentrations which in cell cultures are required to inhibit cell proliferation when exposure times are short. With paclitaxel on the balloons, much higher concentrations were reached. It seems that premounted stents increase the initial concentration, and also increase the resident time of the drug in the arterial wall. Four weeks after treatment, the paclitaxel concentration in a peripheral porcine artery was high enough to explain inhibition of neointimal proliferation24.
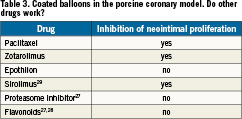
Paclitaxel is known to irreversibly bind to microtubules8,25, and it has been detected in tumour tissue of patients one week after intravenous infusion. Persistent binding to tissue is obviously an inherent property of the paclitaxel molecule, and independent of slow release formulations. Since this slow elimination of a drug from tissue is very exceptional, it is interesting to see if other drugs administered in the same way inhibit neointimal proliferation to a similar extent as paclitaxel does. Only a few drugs have been tested. In a pilot study in pigs, Zotarolimus was efficacious26. Two other cytostatic agents which are much more potent in vitro and following intravenous administration than paclitaxel failed to inhibit neointimal proliferation when coated on balloons (Table 3)27. Anti-inflammatory flavonoids displayed the expected local effect on inflammation, without significantly affecting vessel narrowing27,28. Furthermore, sirolimus-coated balloons were effective in the porcine model29.
In conclusion, the very long lasting persistence of paclitaxel in the vessel wall may explain the inhibition of neointimal proliferation by a single administration of the drug. Provided that the dose is sufficient, a sustained release formulation is dispensable. The exceptionally long persistence of paclitaxel does, however, not exclude the possibility that the decisive action of the drug occurs shortly after administration, and that other drugs with much faster elimination may be equally effective.
Conflict of interest statement
U. Speck and B. Scheller report being named as co-inventors of a patent application by Charité University Hospital, Berlin describing contrast media and drug-coated balloon catheters for local drug delivery. The other authors have no conflicts of interest to declare.
References

