Background
Local delivery of fluid paclitaxel is a stent independent, catheter-based technique for antiproliferative intracoronary pharmacotherapy. The main advantage of this alternative method – as adjunct or alternative to drug-eluting stents – is a homogeneous drug delivery to the whole vessel wall, even between and beyond stent struts without the problems of an additional metal foreign body and coating material as drug carrier.
Our method of local paclitaxel delivery shares this crucial advantage with the paclitaxel coated balloon. It differs, however in one central feature. The technique presented here is not a coated device that requires an angioplasty as prerequisite for drug delivery; it is rather a local pharmacotherapy after PCI with an active drug dissolved in fluid, which is then delivered via a special application catheter.
Since the early 1990s numerous preclinical studies in different species were performed to develop and evaluate this method1-4. We have shown that even a short single dose application of paclitaxel exerts potent and long-lasting antiproliferative effects in vitro and in vivo5. As a result of our preclinical experiments, the combination of a passive atraumatic application catheter with paclitaxel emerged to be the most promising combination of drug and device. On the one hand, the potent pharmacological properties of paclitaxel (lipophilic character, rapid cellular uptake, long lasting action via irreversible alteration of the cytoskeleton) compensate for the low efficiency of a merely passive application system, on the other hand the passive mode of delivery offers the big advantage of being atraumatic to the vascular wall1-7,9-16,18-20,22.
Learning from this experience, we developed, tested and introduced a new application catheter. The so-called GENIE™ catheter is CE-marked, produced by ACROSTAK Corp., Winterthur, Switzerland, and is commercially available21.
GENIE™ local delivery balloon catheter
Basically this system consists of a balloon with a distal and proximal occlusive segment and a central segment (multiple lengths available) that allows for homogeneous transfer of paclitaxel to the vessel wall. In comparison to former local drug delivery devices, this balloon offers the advantage of drug transfer by jets that are directed coaxially to the vessel wall. Holes in the distal ramp of the catheter allow for filling of the drug depot without hydrojets due to an almost parallel flooding of the central segment. The GENIE™ device is based on a monorail PTCA catheter design and is compatible with a 0.014” guidewire and a 5Fr / 0.056” guide catheter. Usable length is 138cm; tip to monorail exit is 23cm. Shaft markers at 90cm, and 100cm respectively, from the tip help in femoral and brachial approach. Lesion crossing profile is from 1.25mm for GENIE™ Ø3.5mm down to 1.05mm for GENIE™ Ø2.5mm. GENIE™ is deployed using standard PTCA techniques. Two radiopaque marker bands help in balloon positioning under fluoroscopy. Total balloon length is 3mm longer than marker band distance; treatment length is about 2mm shorter than marker band distance. Treatment lengths are available in 20, 24 and 28mm21. (Figure 1)
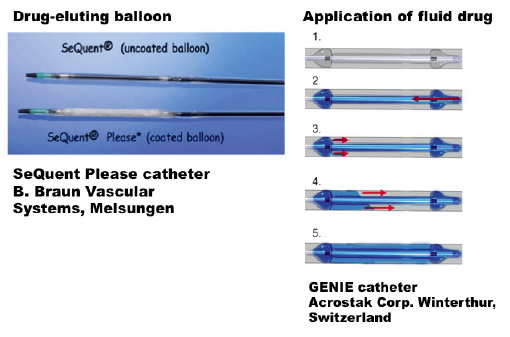
Figure 1. Schematic drawing of drug eluting balloon (SeQuent Please, left) and application catheter for local delivery of fluid drug (Genie, right).
Experimental data with paclitaxel
Paclitaxel (Taxol®) was originally isolated from the bark of the Pacific Yew Taxus brevifolia. It is a diterpenoid with a characteristic taxane-skeleton of 20 carbon atoms and has a molecular weight of 853.9 Dalton. Although the toxicity of extracts from this tree has been known for centuries, it was not until 1964 that the National Cancer Institute in the USA started a screening program for antineoplastic substances and launched the unique pharmacological career of Taxol®8.
Paclitaxel is a potent anti-tumour drug that exerts its pharmacological effects through an unusual mechanism. Unlike other antiproliferative agents of the colchicine type, which inhibit microtubule assembly, it forms numerous decentralised and unorganised microtubules within the cytoplasm by shifting the microtubule equilibrium towards microtubule assembly. These alterations of the cytoskeleton interfere with many functions of the cell, such as proliferation, motility and migration, intracellular transport and transmembrane signalling. Several important biological processes (like the activation of protein kinases) are associated with microtubule depolymerisation; these processes therefore are inhibited by paclitaxel. Furthermore, it is highly lipophilic, facilitating a rapid cellular uptake, and has a long-lasting effect in the cell due to the structural alteration of the cytoskeleton25.
Starting 20 years ago we could show that even a short single-dose application exerts a potent and long-lasting antiproliferative effect on human smooth muscle cells. In dose finding studies, a concentration of 10 µM paclitaxel was chosen, a dose that led to a sustained antiproliferative effect of up to 14 days after single-dose application without cytotoxic effects in cell culture5. This makes paclitaxel a very promising candidate for local drug therapy intended to address the proliferative and migratory processes involved in restenosis. (Figure 2)
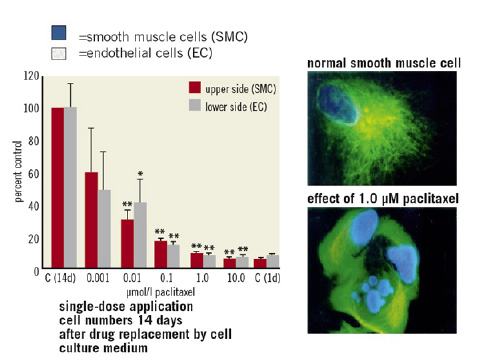
Figure 2. Dose-dependent antiproliferative effect of paclitaxel after short-term single-dose application in cell culture.
The delivery efficiency of different application catheters was evaluated, favouring the passive double-balloon device in an ex vivo experiment with radioactive labelled paclitaxel in freshly explanted porcine hearts9. The next step was to evaluate the effects of locally delivered paclitaxel on proliferation and subsequent neointima formation in vivo in experimental animal angioplasty models. First, the effects of locally delivered paclitaxel were evaluated in a second injury model in the rabbit carotid artery12. After induction of a defined plaque in the right carotid arteries of 76 New Zealand rabbits by electrical stimulation, 27 animals underwent balloon dilatation and subsequent local paclitaxel delivery (10ml, 10µM) with a double-balloon catheter. Twenty-nine animals served as control with angioplasty only, 10 animals underwent local delivery of vehicle only (0.9% NaCl-solution) and 10 animals were solely electro-stimulated. Vessels were excised one, four and eight weeks following intervention. In order to determine the extent of cells undergoing DNA-synthesis, bromodeoxyuridine (BrdU) was given to each animal before the excision of the vessels. Immunohistological staining of cells with incorporated bromodeoxyuridine was performed to identify replicating cells by using amonoclonal antibody against BrdU. The following histomorphometric parameters were measured: luminal area (mm2), neointimal area (mm2), internal elastic lamina area (IEL area; mm2), external elastic lamina area (EEL area; mm2) as ameasure of total vessel area and maximal neointimal thickness. The % area stenosis was defined as neointimal area / IEL area expressed as a percentage. The extent of stenosis in paclitaxel-treated animals was significantly reduced compared to balloon-dilated control animals (p=0.0012, one, four and eight weeks after intervention: 14.6%, 24.6% and 20.5%, versus 24.9%, 33.8% and 43.1%). Marked vessel enlargement compared to balloon-dilated control animals was observed in animals treated with local paclitaxel delivery (p=0.0001, total vessel area after one, four and eight weeks: paclitaxel group: 1.983mm2, 1,700mm2 and 1.602mm2, control: 1.071mm2, 1.338mm2 and 1.206mm2). The number of endothelial cells did not differ significantly among all three study groups, suggesting no considerable delay of re-endothelialisation in the rabbit animal model.
The specific action of paclitaxel on the cytoskeleton allows for aunique feature, the detection of the drug action by staining of tubulin and electron microscopy. In paclitaxel-treated rabbits, tubulin staining showed an increase in fluorescence intensity indicating changes in microtubule assembly. This gave reason for further investigations of the effects of paclitaxel on the vessel wall by electron microscopy. In fact, parallel microtubule bundles could be detected in SMCs that had been exposed to local paclitaxel delivery. It has been shown previously that these parallel orientated microtubule bundles are a typical paclitaxel-induced modification in microtubule assembly. In control animals, no changes in the cytoskeleton were found at any time point10,14.
From these data it followed that local catheter-based delivery of paclitaxel led to a reduction of restenosis formation by synergistic effects on proliferation and remodelling, mediated by structural changes of the cytoskeleton of vascular smooth muscle cells10,12-14,25. Finally, these encouraging results had to be reproduced in an intracoronary experimental animal model, which is why we performed another animal study in pigs22.
In this animal study, 30 domestic pigs were included. All animals received either bare metal stent implantation (n=15) or balloon angioplasty (n=15). In 10 pigs local delivery of the paclitaxel solution via the GENIE catheter was performed, 10 animals served as asham group and received vehicle (0.9% NaCl solution), and 10 animals were used as a control group without local delivery. All animals were on aspirin and clopidogrel to prevent stent thrombosis. After final angiography the vessels were excised six weeks after intervention and further prepared for histological/histomorphometric analysis. After excision, coronary arteries were dissected from the heart and perfusion-fixed with PBS (phosphate buffered saline) and paraformaldehyde. The segments were embedded in 2-hydroxyethylmetacrylate or paraffin. Stented segments were sectioned into 200µm slices with the struts remaining in situ or 4µM slices (without stents) and then stained with hematoxilin-eosin solution or Elastica van Gieson staining. All coronary arteries showed complete endothelialisation 42 days following treatment (stent implantation or balloon dilatation). Paclitaxel treatment led to a marked reduction of parameters characterising restenosis either post-stent implantation (neointimal area: 1.04±0.10mm2 in paclitaxel-treated animals versus 2.33±0.25mm2 in sham controls and 2.37±0.25mm2 in stented control animals; p<0.001) or post-balloon dilatation (neointimal area: 0.35±0.14mm2 in paclitaxel-treated animals versus 0.71±0.26mm2 in sham controls and 0.68±0.24mm2 in solely balloon-dilated control animals; p<0.01). There were no significant angiographic or histomorphometric differences between the control and the sham group. In both paclitaxel groups neither edge phenomena nor a significant inflammatory response occurred in the treated vessel segments. No side effects were detected in all of our animal studies, as measured with clinical monitoring (ECG, blood samples, haemodynamic, follow-up); however, these side effects cannot be generally excluded. Yet again, local delivery of 10ml 10µM paclitaxel will not even result in measurable systemic plasma levels in humans. After positive results in different species, and numerous different preclinical studies, we therefore felt it safe enough to test this method in a randomised clinical trial in humans.
Clinical evaluation
Between August 2005 and August 2007, we conducted a prospective, randomised trial comparing the local delivery of a liquid paclitaxel preparation after bare metal stent implantation (groupI) with the implantation of a bare metal stent (BMS) without subsequent administration of paclitaxel (groupII, negative control) and the implantation of a paclitaxel eluting stent (groupIII, positive control) in 204 patients with symptomatic coronary artery disease (CAD). The primary endpoint was angiographically defined in-stent and in-segment late lumen loss (LLL). Secondary endpoints included a binary restenosis rate of >50 %, minimal lumen diameter (MLD) and diameter stenosis. Various safety parameters including 30-day major adverse cardiovascular event (MACE) rate were evaluated. We also analysed a composite clinical endpoint consisting of death, myocardial infarction and percutaneous or surgical revascularisation of the target lesion (TLR) six months after intervention.
At six months, angiography showed an in-stent LLL of 0.61±0.44mm in group I versus 0.99±0.72mm in group II (I vs. II, p=0.0006) and 0.44±0.48mm in group III (non-inferiority of I vs. III, p=0.023). The cumulative overall rate of major cardiac events was 13.4% in group I, 26.8 % in groupII and 14.9% in groupIII. TLR rate was 13.4 % (I), 22.1 % (II), and 13.4 % (III). From these results we followed that the additional antiproliferative treatment of de novo lesions in native coronary arteries with catheter-based delivery of a paclitaxel solution was safe and significantly reduced neointimal proliferation, restenosis and clinical events compared to BMS implantation alone. Compared to the implantation of a DES, catheter-based delivery of paclitaxel was not statistically inferior26.
Encouraged by the positive results of the LOCAL TAX study, patients are increasingly treated with this procedure in clinical routine practice. Among these patients are many who were ultimately scheduled for CABG surgery, such as patients with DES failure or in-stent bifurcation restenosis. Patients are included in different registers at our institution (in-stent restenosis, chronic total occlusion, bifurcation stenosis and DES failure)23,24.
In conclusion, catheter-based intracoronary delivery of fluid paclitaxel using a special “low pressure” application balloon is technically easy and feasible without procedural complications. Specific local or systemic side effects were not documented in nearly 200 patients up to 5-years after intervention. The safety and efficacy data of this new approach are encouraging and give hope that an additional technique of local intracoronary pharmacotherapy (besides drug-eluting stent or drug-eluting balloon) can be established in clinical routine.
The concept of delivering drugs in solution with atraumatic catheters provides major flexibility in two crucial aspects: first, the possibility to deliver various compounds or even a combination of different pharmacological principles; in fact, preliminary preclinical data with an antithrombotic compound were promising20. Second, the possibility to treat longer or more complex vessel segments medically without mechanical trauma. Using this technique new indications emerge –including a preventive pharmacotherapy of critical lesions without any mechanical intervention in near future.
Case reports from the LOCAL TAX study program
LOCAL TAX study
A 71 year-old patient who had two significant stenoses in the right coronary artery. Two identical bare metal stents were implanted, afterwards the distal lesion was treated with local delivery of fluid paclitaxel. At the 6-months angiographic follow-up, the patient had a significant restenosis in the proximal untreated bare-metal stent (segment 2) and no restenosis in the distal paclitaxel-treated distal lesion (segment 3). (Figure 3)
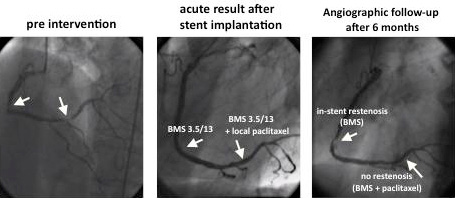
Figure 3. LOCAL TAX study program. Bare metal stent (BMS)+local paclitaxel delivery. Example from LOCAL TAX study: patient Nr.3- E.S. (71 years, male)
LOCAL TAX II (ISR) registry
A 57 year old patient who presented with subacute myocardial infarction. In the acute situation, for some unreported reason, he was treated only with PTCA and no stent implantation. Seven months later he was treated with bare metal stent implantation because of restenosis. Again, three months later he had in-stent restenosis and was treated with a Cypher stent. An additional four months later he had diffuse restenosis in a long segment of the RCA. At this time he received a Taxus stent and again, four months later, he presented with repeat-repeat-repeat restenosis. At that time, presenting with three layers of metal in his RCA, CABG surgery would have been the logical recommendation. However, because surgery was not an option for the patient, local paclitaxel delivery of the whole vessel was performed. Angiographic controls three and nine months after this final procedure showed no new restenosis. The patient has remained event-free for more than three years until the present time23. (Figures 4 and 5)
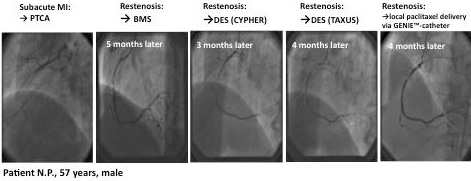
Figure 4. LOCAL TAX II (ISR) register. Example: PTCA+local paclitaxel delivery.
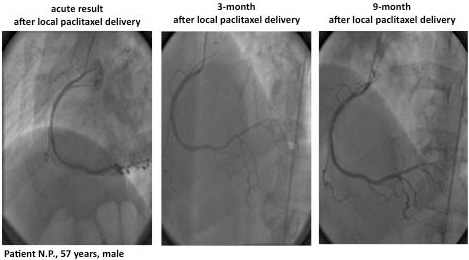
Figure 5. LOCAL TAX II (ISR) register. Example: PTCA+local paclitaxel delivery.
Even though the LOCAL TAX II (ISR) is just a registry and not a randomised study, the results seem quite convincing. There are 87 patients so far that completed 3-year follow-up, 37 of which had drug-eluting stent failure. Ten patients underwent target vessel revascularisation. One patient died of cholangiocellular carcinoma and 75 patients stayed clinically and angiographically event-free.
LOCAL TAX III (CTO)
A 76 year old patient presented with a functionally occluded right coronary artery and signs of ischaemia in a positive stress test. He ended up with six bare metal stents (diameter 2,5mm, total stent length: 74mm). Immediately afterwards he was treated with fluid paclitaxel delivery for the whole artery. Nine months later he presented with a focal restenosis in segment 2 of the RCA. Again, he was treated with local paclitaxel delivery following PTCA (no stent). Since then he has remained clinically event-free for three years. At 9-month angiographic follow-up, the final result was still good with no need for re-intervention. (Figure 6)
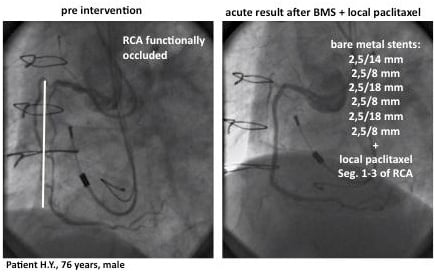
Figure 6. LOCAL TAX III (CTO) register. Example: BMS+local paclitaxel.
LOCAL TAX IV (Bifurcations)
Two GENIE catheters in used in with the kissing balloon technique is an elegant way of treating bifurcation lesions24. Local delivery of fluid paclitaxel using the kissing balloon technique allows for medical treatment of the whole bifurcation without adding new metal to a lesion that is difficult to treat with stents per se. Especially with in-stent bifurcation restenosis, it is often impossible to add additional layers of metal to the lesion –therefore this method offers aunique way of treating interventionally even these patients with asimple and effective medical therapy. (Figure 7)
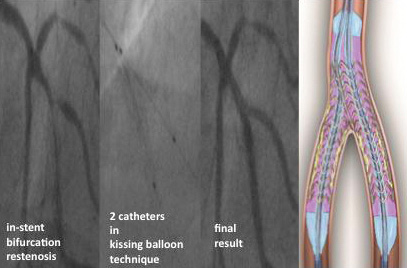
Figure 7. LOCAL TAX IV (bifurcation) register. Example: “kissing balloon” technique.
Conflict of interest statement
Dr. Herdeg is a consultant for Boston Scientific.
References

