Abstract
Aims: To evaluate effects of sirolimus-eluting stents (SES) compared to bare-metal stents (BMS) at stent edges in patients with acute myocardial infarction (AMI).
Methods and results: Clinical, angiographic, intravascular ultrasound (lVUS) and virtual histology (VH)-IVUS results were obtained and analysed in 20 SES and 20 BMS AMI patients at the index procedure and at nine months follow-up. Quantitative angiography and IVUS showed a trend toward decreases in mean lumen diameter, vessel volume, minimum lumen area and mean lumen area at both stent edges of BMS, and at the proximal edge of SES. At the distal stent edge, a significant difference between BMS and SES treated patients in mean lumen area was found (∆-0.8±1.6 mm2 versus ∆0.2±0.8 mm2 respectively, p=0.04). Furthermore, in-stent SES had a larger lumen volume (SES: 167.7±59.2 mm3 versus BMS: 125.1±43.8 mm3; p=0.02) and less neointima volume (7.3±9.1 mm3 versus 53.2±35.1 mm3; p<0.001). Neither SES nor BMS demonstrated a significant effect on plaque composition at follow-up VH-IVUS analysis.
Conclusions: A significant difference between SES and BMS treated patients was observed with respect to mean lumen diameter distal to the stented segment which suggests a downstream effect of sirolimus elution.
Abbreviations
AMI: Acute myocardial infarction
BMS: Bare-metal stent
CRP: C-reactive protein
CSA: Cross-sectional area
EEM: External elastic membrane
IVUS: Intravascular ultrasound
Mean LD: Mean lumen diameter
MLD: Minimum lumen diameter
PTCA: Percutaneous transluminal coronary angioplasty
QCA: Quantitative coronary angiography
SES: Sirolimus-eluting stent
STEMI: ST-segment elevation myocardial infarction
VH-IVUS: Virtual histology intravascular ultrasound
Introduction
The treatment of patients with acute myocardial infarction (AMI) changed significantly over the last decades. Early pharmacological and, more recently, mechanical reperfusion strategies improved the prognosis of AMI patients further supported by optimal medical treatment (including antiplatelet therapy, ACE inhibitors, beta blockers and statins) as well as life style changes in the chronic phase after the acute event. Procedural outcome also improved due to improved operator experience, continuous refinement of catheter and balloon technology, and the introduction of intracoronary stents. More recently, drug eluting stents (DES) have been introduced. Although the efficacy of DES is proven in patients with stable coronary artery disease, the role of DES in AMI patients is still under debate1-4. Despite the positive effects of DES on restenosis, the increased risk of subacute thrombosis tempered the initial enthusiasm as subacute stent thrombosis is a devastating event associated with high mortality rates and myocardial infarction5-9. In AMI patients the initial event is caused by disruption or erosion of a vulnerable plaque10-12 leading to acute thrombosis. The increased risk of stent thrombosis associated with DES is caused by incomplete/delayed neointimal coverage and may be prevented to some extent in most patients with stable coronary artery disease by prolonged dual antiplatelet therapy. Although after implantation of a stent the ruptured plaque will be covered, it is unclear what happens proximal and distal to the stent segments. Some studies suggest that the eluted drug in the case of a DES may not only have an effect on the stented segment, but also on adjacent segments as well13-15. It is therefore of interest to study these segments during the initial procedure and during follow-up. In this study we evaluated the effects of DES compared to bare-metal stents (BMS) on the proximal and distal segments using intravascular ultrasound imaging (IVUS) in AMI patients at baseline and at nine months follow-up. Although IVUS allows cross-sectional imaging of coronary arteries and provides a comprehensive assessment of the atherosclerotic plaque, it cannot provide detailed data about its tissue components. Detecting changes in tissue components may increase our comprehension of in vivo development of potentially vulnerable plaque. Therefore, in addition, virtual histology (VH-) IVUS, using spectral analysis of the radiofrequency ultrasound backscatter signals to analyse plaque composition and morphology was used. VH-IVUS allows identification of four different components of atherosclerotic plaques: fibrous, fibro-fatty, dense calcium, and necrotic core16.
Methods
Study design and population
Patients for this substudy were selected from the randomised MISSION! intervention study (current controlled trials number, ISRCTN62825862,4). The original MISSION! study was designed to compare the outcome of the sirolimus coated Cypher stent (Cypher Select™, Cordis Corp., Miami Lakes, FL, USA) with the bare-metal Vision stent (Multilink Vision™, Guidant Corp., Indianapolis, IN, USA) in patients with AMI4. The study was approved by the institutional ethical committee. Written informed consent was obtained from all patients before enrollment and before the follow-up catheterisation at nine months. The study protocol, inclusion and exclusion criteria, endpoint definition and main outcomes of the study were published previously4.
For this substudy, a group of consecutive patients were included for whom not only clinical, angiographic and IVUS data were performed, but for which also VH analysis was performed from both the index procedure and the nine months follow-up study. Throughout the study period all patients were treated according to the institutional STEMI protocol, which included standardised out-patient follow-up17. In brief, all patients had symptoms of STEMI that started <9 hours before arrival at the catheterisation laboratory and an ECG that demonstrated a STEMI (ST segment elevation ≥0.2 mV in ≥2 contiguous precordial leads [V1-V4], or ≥0.1 mV ST elevation in other leads, or a new left bundle branch block).
Key exclusion criteria were age <18 years or >80 years; the presence of a left main lesion of ≥50% stenosis; triple vessel disease, defined as ≥ 50% stenosis in three major epicardial vessels; previous percutaneous coronary intervention or bypass grafting of the culprit vessel; failed thrombolytic therapy for the index infarction; reference diameter of the culprit lesion of less than 2.25 mm or larger than 3.75 mm; and lesion length ≥ 24 mm.
Procedure protocol
Before the index procedure all patients received 300 mg of aspirin, 300-600 mg of clopidogrel, and an intravenous bolus of abciximab (25 µg/kg), followed by a continuous infusion of 100 µg/kg for 12 hours. At the start of the procedure 5000 IU of heparin was administered. Coronary lesions were treated according to current interventional practice. If more than one stent was required, the additional stent was of the same assigned study type.
After intervention IVUS imaging was performed to document angiographic result. Each angiogram and ultrasound sequence was preceded by 200-300 µg of intracoronary nitroglycerin.
After the procedure aspirin (100 mg/day) was prescribed indefinitely and clopidogrel (75 mg/day) for 12 months. During follow-up, patients were treated with beta blockers, statins and ACE-inhibitors or ATII-blockers, according to current guidelines17. Patients were seen at the out-patient clinic at 30 days, 3, 6, and 12 months. Follow-up angiography and (VH-) IVUS image acquisition was performed at nine months follow-up.
Quantitative coronary angiography (QCA)
Coronary angiograms obtained at baseline and at nine months follow-up were digitally recorded and analysed, blinded to the assigned treatment.
The analysis was performed using automated edge-detection software (CMS version 6.0, Medis Medical Imaging Systems, Leiden, The Netherlands) at a single projection showing the most severe stenosis. The same projection was used at follow-up. The proximal and distal edges were evaluated up to 5 mm from the stent.
IVUS analysis
IVUS imaging was performed with motorised pull-back (0.5 mm/s) starting at least 10 mm distal to the stent, ending at the coronary ostium. A 2.9 Fr 20 MHz catheter and a dedicated IVUS console (Eagle Eye, Volcano Corp. Rancho Cordova, CA, USA) were used18. Before imaging, 200-300 µg of intracoronary nitroglycerin were administered. Analysis was performed by analysts that were blinded to the assigned treatment using customised software (QCU-CMS 4.14, Medis, Leiden, The Netherlands) for analysis of the quantitative greyscale data.
External elastic membrane (EEM) cross sectional area (CSA) and lumen CSA of segments 5 mm proximal and distal to the stent were determined per frame and vessel volume, mean lumen area and minimal lumen area for these segments were compared to the same parameters at follow-up.
Virtual histology analysis
VH images were generated simultaneously during motorised pull-back (Figure 1). Images were acquired at every R-peak during continuous ECG registration. Data were stored digitally on CD for off-line analysis. Atherosclerotic coronary lesions were characterised by classification trees based on mathematical autoregressive spectral analysis of IVUS backscattered data (pcVH software version 2.2, Volcano Therapeutics; Volcano Corp., Rancho Cordova, CA, USA). Fibrous areas were marked in green, fibro-fatty in yellow, dense calcium in white and necrotic core in red on the reconstructed colour-coded tissue map. The area and volumes of each plaque component were calculated automatically by the pcVH software. PcVH analysis software is commercially available image analysis software developed by Vocano Therapeutics and the technique has been validated in past histopathological validation studies of VH-IVUS19,20.
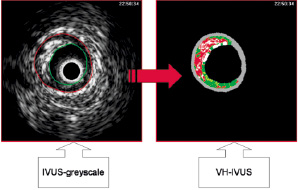
Figure 1. Illustration of creating an image with virtual histology intravascular ultrasound. IVUS: intravascular ultrasound; VH-IVUS: virtual histology intravascular ultrasound.
Measurements were made for the region of interest, which was defined as the segment of minimal 5 mm to maximal 10 mm distal and proximal to the stented segment. This range was chosen instead of the conventional 5 mm distance because: 1) it was unclear, assuming that there would be a downstream effect of the drug on plaque composition, how far the effect would reach and 2) as it was expected that these effects were likely to be small, it was considered preferable to include as much potentially affected longitudinal distance as possible. Catheterisation images of the index procedure and of nine months follow-up were analysed side-by-side to ensure that the same segments were studied.
Although the volumetric analysis of the software could not be adjusted for repeated frames (implicating that the volume analysis of the same segment at different points of time could slightly differ), this problem was solved by using relative plaque volumes for final analysis (percentage of total plaque). Repeated frames were caused by the catheter getting stuck during pullback. In the 40 cases presented in this study only a small number of such frames were observed. Nevertheless, to minimise their influence on results for absolute plaque areas, repeated frames were ignored during this analysis.
Statistical analysis
Continuous data are reported throughout this text and in the tables as mean ± standard deviation. Evenly distributed continuous data were analysed by utilising the independent sample t-test. Unevenly distributed continuous data were analysed using an equivalent non-parametric test and the Mann-Whitney U test. For comparison to follow-up, analysis of continuous data at different points in time was performed by the paired Student’s t-test. Categorical data are summarised as proportions and were compared with Pearson’s X2 -test or Fisher exact test in case of one or more cells in the contingency table with expectation less than 5, as appropriate. All tests were two-sided, a p-value of <0.05 was considered significant.
Results
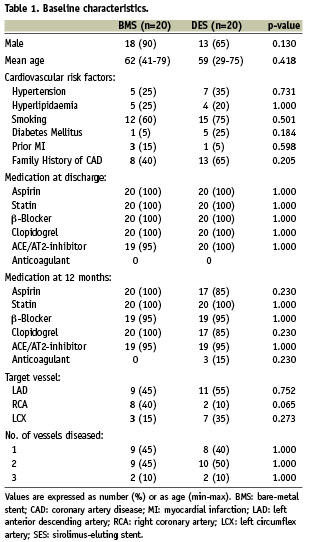
Forty patients who received a SES (n=20) or BMS (n=20) during a primary PTCA were included in this substudy. There were no significant differences in baseline patient characteristics between the two patient groups (Table 1).
Angiographic results
Index-procedure and follow-up QCA-results for minimum lumen area and mean lumen diameters are reported in Table 2. In BMS patients, both minimum and mean lumen diameter decreased at both sides of the stent at follow-up though this did not reach statistical significance at the distal stent edges (MLD proximal edge: from 2.76±0.42 mm to 2.62±0.46 mm; p=0.03 and Mean LD proximal edge: from 3.05±0.48 mm to 2.94±0.46 mm; p=0.03, MLD distal edge: from 2.43±0.48 mm to 2.21±0.67mm; p=0.10 and Mean LD from 2.65±0.55 mm to 2.48±0.69 mm; p=0.19).
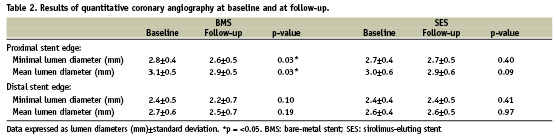
In SES patients a similar (although non-significant) decline in lumen area was observed at the proximal stent edge, the distal MLD and Mean LD however tended to increase (MLD) or remained unchanged (Mean LD) during follow-up (MLD proximal edge: from 2.74±0.37 mm to 2.68±0.46 mm; p=0.40 and Mean LD from 3.04±0.57 mm to 2.94±0.57 mm; p=0.09, MLD distal edge: from 2.38±0.39 mm to 2.44±0.53 mm; p=0.41 and Mean LD from 2.61±0.37 mm to 2.61±0.5 mm; p=0.97).
IVUS greyscale results
Quantitative post-procedural and follow-up IVUS data are summarised in Tables 3 and 4. At the proximal stent edge, vessel volume and lumen areas decreased in both BMS and SES patients at nine months follow-up (BMS: –3.6% and –0.5% and SES: –7.2% and –8.2%). The mean lumen area decreases significantly in the SES group at the proximal stent edges (p=0.03).
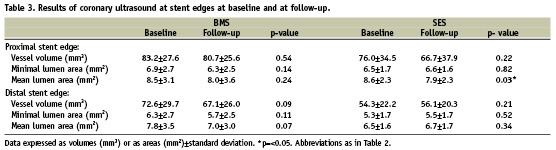
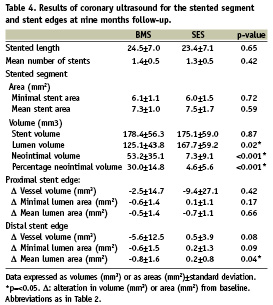
At follow-up, vessel volume and mean lumen area of the distal stent edge of the BMS group tended to decline (overall decrease of –5.0% and –6.3% respectively, p=ns), while they increased in the SES group (vessel volume increase of 1.5% and mean lumen area increase of 3.4%, p=ns). There were no significant differences between BMS and SES groups in vessel volume and mean lumen area changes except for mean lumen area at the distal stent edge (∆–0.8±1.6mm2 versus ∆0.2±0.8 mm2 respectively, p=0.04; Table 4). Within the stented segment however, the SES group demonstrated a significantly larger lumen volume (SES: 167.7±59.2 mm3 versus BMS: 125.1±43.8 mm3; p=0.02) at follow-up. Also in SES, less neointima volume (7.3±9.1 mm3 versus 53.2±35.1 mm3; p<0.001) and lower percentage neointimal volume (4.6±5.6% versus 30.0±14.8%; p<0.001) were found at follow-up when compared to BMS.
Virtual histology results
Post-procedural and follow-up VH-IVUS results for relative volumes of the different plaque components are reported in Table 5. The relative increase and decrease of each plaque component volume is illustrated in Figure 2. Except for a significant decrease of the fibrous plaque component volume of the distal edge segment of BMS (p=0.05), plaque composition did not change significantly in either group. The data for mean areas at baseline and follow-up are summarised in Table 6 and the relative increase/decrease of mean area of every plaque component at follow-up is depicted in Figure 3. Again, no significant difference in mean area of the four plaque components is discernible between follow-up and baseline in either group.
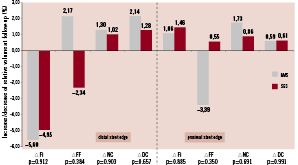
Figure 2. Relative volume changes over nine months time (∆%). Measurements for BMS are set out next to SES. P-values are reported to indicate statistical significance of differences in plaque component changes between BMS and SES. None are significant. BMS: bare-metal stents; SES: sirolimus-eluting stents; ∆: change in relative volume of mentioned plaque component; Fi: fibrous; FF: fibro-fatty; DC: dense calcium; NC: necrotic core
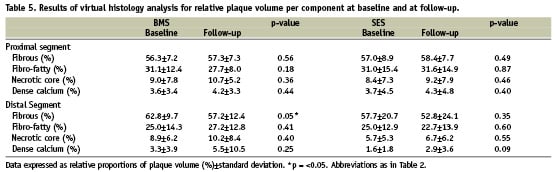
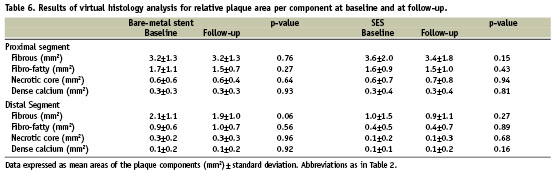
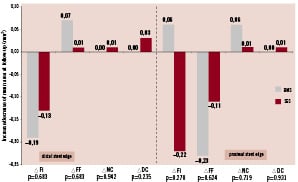
Figure 3. Mean area changes over nine months time. Measurements for BMS are set out next to SES. P-values are reported to indicate statistical significance of differences in plaque component changes between BMS and SES. None are significant. ∆ mm2: change in mean area of mentioned plaque component; Other abbreviations as in Figure 2.
As it was considered possible that by using the calculated means of data from the edge segments any differences between baseline and follow-up in both stent type groups may have been obscured, it was decided to perform an additional VH-IVUS analysis of the frame just distal from the stent edge at baseline and at follow-up for all 40 cases. However, this analysis too revealed no significant differences for plaque composition areas between post-intervention and follow-up and between the two stent types (not shown).
Clinical outcome
One BMS patient and one SES patient underwent a target lesion revascularisation due to restenosis. No patient died during the follow-up period of 12 months. Three BMS patients and two SES patients underwent revascularisation of a vessel other than the culprit vessel at different points of time within a 12 months follow-up period. Adherence to medication was high (Table 1), no sub-acute thrombosis was observed.
Discussion
To our knowledge, this is the first follow-up study that compares vascular plaque composition and remodelling at stent edges between BMS and SES treated patients. The main findings of this study are: 1) at distal and proximal stent edges in BMS patients there is a trend towards negative vascular remodelling, while there is a trend towards positive remodelling at the distal stent edges in SES treated patients resulting in a significant difference between the two groups; 2) plaque composition at the stent edges did not change significantly during the nine months follow-up in either SES or BMS patients.
Sirolimus-eluting stent implantation results in a significant reduction of restenosis compared to the results obtained with bare-metal stents in patients with stable and unstable angina14,21. However, inconsistent and limited data have been presented about their safety and efficacy in patients with acute myocardial infarction1-4. Sirolimus is a potent anti-inflammatory, immunosuppressive and antiproliferative drug effective in inhibiting in-stent neointimal hyperplasia22. These potent antiproliferative effects can also induce positive remodelling and stent malapposition and may cause deleterious local phenomena such as necrosis or apoptosis4,13,22. These effects may potentially affect plaque composition behind the stent, the vessel wall, and as a result of downstream effects of the eluted drug may also affect plaque composition at the distal stent edge.
Clinical data support the hypothesis of drug elution distal to the stent. In recent trials, SES implantation resulted in higher restenosis rates at the proximal edge of the stent compared to the distal edge14. One expects the concentration, and therefore the effects of the drug, to be strongest in the direction of the blood flow. This seems to be confirmed by a study by Degertekin et al showing a trend towards positive remodelling only at the distal stent edge but not at the proximal edge13. Comparative results have been found in several other studies. Investigators of the RAVEL trial reported a trend toward larger lumen areas at distal edge which was thought to be due to higher downstream effect of the drug. A study from Jimenez-Quevedo et al further confirmed these findings by reporting a significant increase in lumen dimensions at stent edges of SES compared to lumen reduction at stent edges in BMS in patients with diabetes22,23. Our findings are in agreement with this, as we observed a trend towards positive remodelling at the distal stent edges in SES patients, though, similar to Degertekin et al, not statistically significant.
Several studies reported that stent edge burden is an important periprocedural predictor of stent edge restenosis after BMS and SES implantations24-26. In the present study population, the stent edge plaque burden may have been too small to be affected significantly by the drug.
Thus far, effects of sirolimus on proximal and distal stent edges have not been fully evaluated. Serruys et al reported a vascular response at the proximal and distal stent edges after paclitaxel-eluting stent implantation15. The paclitaxel-eluting stent induced positive remodelling 1 mm proximal and 3 to 4 mm distal to the stent edges which resulted in less late luminal loss compared to BMS.
Little is known about the effects of sirolimus on the plaque composition at the proximal and distal stent edges. Our findings suggest that plaque composition at the stent edges was not affected by the drug since no significant differences could be detected between BMS and SES patients at follow-up. This is interesting given that an effect of sirolimus on vascular lumen dimensions was clearly present distal to the stent as well as a detectable effect on neointima volume inside the stent as demonstrated by the IVUS greyscale data. As expected, within the stented segment, SES was associated with significantly less neointimal hyperplasia when compared to BMS, a well known effect4,22. At the stent edges however, a decrease in local drug delivery may have occurred, which may have caused the drug to be ineffective in inducing changes in plaque composition at stent edges. However, changes in plaque composition caused by the drug may be subtle and missed by the VH-IVUS technique.
Similar data were observed in a study from Aoki et al, who reported in a long-term follow-up study of 23 event-free patients treated with SES that no significant changes in plaque echogenicity at the distal stent edges (5 mm) had taken place across multiple time points of follow-up27. At the same time, investigators did see a change of plaque echogenicity behind the stent struts between two years and four years of follow-up. This is interesting, as this suggests that alterations in plaque composition take place very late (> 2 years) after stent implantation and that they are very localised, therefore possibly not involving a measurable downstream edge effect.
It may be that the remodelling effect on the vessel at the distal stent edge is a general effect that is caused merely by a delayed healing response. Caramori et al demonstrated persistent vasomotor dysfunction distal to coronary stents implanted six months earlier28. The antiproliferative effect of sirolimus may merely cause a prolonged healing response with concomitant delayed recovery of endothelial function as suggested by Hofma et al29. It has been suggested before that delayed vascular healing may cause positive remodelling and incomplete stent apposition30,31.
In addition, past studies suggested that sirolimus or the polymer might induce apoptosis or necrosis32,33. It was suggested that the strong hydrophobic property of the compound partitions highly into arterial tissue resulting in drug concentrations that exceed the applied bulk concentration33,34. This highly concentrated local delivery of a potent drug may lead to increased vascular toxicity which in turn may lead to an inflammatory response. Pires et al, investigated histopathological effects of sirolimus- and paclitaxel-eluting cuffs in a murine model for restenosis on underlying diseased atherosclerotic arteries33. While paclitaxel significantly increased apoptosis, internal lamina elastic disruption, and decreased medial and intimal smooth muscle cells and collagen, vascular histopathological analysis revealed that sirolimus had no significant adverse effects on vascular pathology. This further supports our finding of unaffected plaque composition in SES.
Limitations
This study is limited by the fact that it was a single-centre study and that, due to the complex nature of the study design, the patient sample size for which all the above mentioned imaging modalities were available was relatively small4. Nonetheless, though it may not be possible to firmly conclude that no difference of effect on plaque composition exists between SES and BMS on stent edges after nine months of follow up, the data indicates that these differences are possibly of smaller magnitude than anticipated. The relatively short follow-up of nine months may have been a possible limitation, as changes in plaque composition may take much longer to develop than was presumed. Larger and longer follow-up studies of well-matched patient populations will be able to tell just how much of a difference there truly is.
Secondly, using VH-IVUS analysis at the index procedure made the border detection a more complex process35,36. Inaccurate detection of borders shared by thrombus, plaque and lumen might have caused measurement errors of plaque composition.
Conclusion
This study demonstrates a trend towards positive remodelling at the distal stent edges in SES patients and a significant inhibition of neointimal hyperplasia within the stented segment at follow-up as compared to BMS treated patients. The effect on the distal stent edge suggests a downstream effect of sirolimus elution despite the fact that an effect on plaque composition was not observed.

