Abstract
Aims: Stent length is a major predictor of restenosis in stable patients undergoing percutaneous coronary intervention (PCI) with bare metal stents (BMS). The effect of stent length is decreased by using drug eluting stents, however, this association had not been previously determined in patients with acute myocardial infarction (AMI). We sought to determine the impact of stent length on restenosis in patients who undergo primary PCI for AMI.
Methods and results: Three-hundred and fifty-seven and 355 patients with AMI were included respectively in the BMS and SES (sirolimus eluting stents) arms of the Trial to Assess the Use of the Cypher Stent in Acute Myocardial Infarction Treated with Balloon Angioplasty (TYPHOON). Patients were divided into four subgroups based on the total length of the culprit lesion stented segment (in mm) : <18, ≥18 and <23, ≥23 and < 28, and ≥28 (groups 1 – 4 respectively). Target lesion revascularisation (TLR) and angiographic late loss were used to assess the restenotic process. Despite similar lesion length, average stent length was longer in patients treated with SES as compared to BMS 22.1±8.6 and 20.3±8.2 mm respectively, p=0.005. The rate of 12m death and AMI was similar in SES and BMS. There was no significance influence of stent length on % TLR neither in BMS (12.6, 10.1, 17.4 and 12.3 – subgroups 1-4 respectively) nor in SES (3.9, 5, 2.2 and 2.7 respectively). There was also no significant impact of stent length on angiographic late loss (mm) neither in BMS (0.7, 0.87, 0.84 and 0.92 respectively) nor in SES (0.32, 0.0, 0.11 and 0.3 respectively).
Conclusions: Physicians tend to choose longer SES than BMS for a similar lesion length during primary PCI for AMI. Interestingly, stent length did not affect clinical or angiographic restenosis neither in BMS nor in SES in this group of patients who underwent primary PCI for acute MI. This data challenges current practice concerning the chosen stent length in patients with AMI.
Introduction
Stent length is considered one of the major predictors of restenosis after PCI1-4. The relation between stent length and restenosis is well documented with BMS while the clinical significance of this relation with SES is much less important. Physicians, thus, tend to adopt a strategy in which longer drug eluting stents (DES) (as compared to BMS) are used to treat coronary lesions of a similar length. Data to support the above strategy was derived from analysis of trials that did not include patients with AMI. We sought to determine whether a strategy of using longer SES as compared to BMS is also justified during primary PCI for AMI. We used quantitative angiographic and clinical data from TYPHOON5 to examine the impact of stent length on restenosis in the setting of primary PCI for AMI. Data were analysed for patients with BMS and SES.
Materials and methods
Study population
TYPHOON included 712 patients with ST segment elevation AMI treated with primary PCI at 48 medical centres. Briefly, this was a randomised, single-blind evaluation of the sirolimus-eluting Bx Velocity™ stent (Cordis, Johnson & Johnson, Warren, NJ, USA) versus standard BMS5. Patients were eligible for the trial if their symptoms began less than 12 hours before catheterisation, and if the electrocardiogram showed ST segment elevation. Major exclusions included: the administration of fibrinolytic agents for the index infarction, previous PCI of the infarct-related vessel, overt acute heart failure, previous AMI, and an estimated life expectancy of less than 12 months. The study protocol was approved by the ethics committee at each participating institution and was conducted according to the principles of the Declaration of Helsinki.
Randomisation was performed after visualisation of the detailed morphology of the culprit lesion. It was thus performed after diagnostic angiography in patients with patent arteries and after the establishment of flow (after passage of angioplasty wire and, when required, angioplasty balloon) in those with an initially occluded artery. Criteria for angiographic exclusion included excessive tortuosity or calcification, ostial or multiple lesions, massive thrombus, bifurcation or left main coronary artery disease, and severe multivessel disease requiring surgical revascularisation. Patients were finally included and randomly assigned to a treatment group if the target lesion had a maximum length of 30 mm and was located in a native coronary artery with a reference vessel diameter of 2.25 mm to 3.50 mm. Operators were not blinded to the type of stent utilised (and various BMS’s were allowed) so that they could use the BMS they use in daily practice.
Quantitative coronary angiography
Technicians at an independent angiographic core laboratory (Bio Imaging, Leiden, The Netherlands), who were unaware of treatment assignment, analysed all angiographic images with the use of edge-detection techniques6. Flow in the infarct-related vessel was graded according to the Thrombolysis in Myocardial Infarction (TIMI) trial classification7. Angiographic follow-up at eight months was planned for 200 patients at 12 centres.
Definitions
The primary endpoint of the study was target vessel failure (TVF), defined as the composite of target vessel revascularisation, recurrent infarction, or target-vessel related death at one year. The clinical events committee reviewed and adjudicated all serious clinical events, including stent thrombosis. Target-vessel revascularisation was defined as repeated PCI or bypass grafting of the target vessel, driven by clinical ischaemic symptoms, a positive stress test, or an in-lesion diameter stenosis of more than 70% (visual estimate). TLR was defined as revascularisation due to stenosis of the stented lesion.
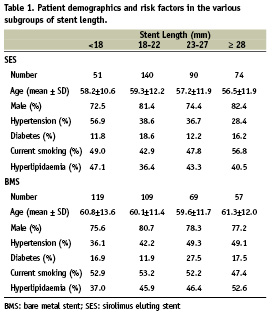
To determine the impact of stent length the BMS and SES groups were divided into four subgroups based on the total length of the culprit lesion stented segment (in mm) : <18, ≥18 and <23, ≥23 and < 28, and ≥28 (subgroups 1 – 4 respectively). Table 1 details the demographic and risk factors of patients in the various subgroups.
Statistical analysis
The impact of stent type was determined by comparisons within each length subgroup. Comparisons among subgroups of stent length were performed using Chi-Square and ANOVA (ordinal and continuous variables) and Chi-Square and T-test were used to compare among BMS and SES. Statistical significance was considered as p<0.05.
Results
Despite similar lesion length, average stent length was longer in patients treated with SES as compared to BMS (22.1±8.6 and 20.3±8.2mm respectively, p= 0.005). Lesion lengths increased with increasing stent lengths subgroup (BMS and SES); however, for the same subgroup lesion length was similar for BMS and SES (Table 2). Longer SES’s were used as reflected by the distribution of numbers of patients in each of the subgroups (Table 2, p<0.001). As expected, stent length increased with lesion length and the mean difference in length among group one and group four was > 20 mm (SES and BMS). Direct stenting was attempted (and actually performed) at decreasing frequency (p<0.001) with the increase in stent length with no difference between SES and BMS (Table 2).
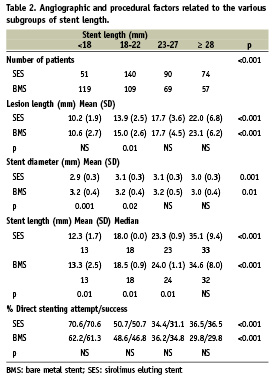
There was no stent length related difference in procedural success and in-hospital and 1-year complication rates (SES and BMS). TVF at one month was similar in BMS and SES and was not influenced by stent length (Table 3). TVF at 12 months was 41% lower in SES than in BMS (8.5% vs. 14.4%, p<0.001) with a similar reduction among the various length subgroups. Similarly, 12 months TLR (a more direct measure of the restenotic process) was reduced by 71% in patients treated with SES (3.7% vs. 12.7%, p<0.001). Again, there was a similar reduction among the different length subgroups. Neither TVF nor TLR at 12 months were influenced by stent length (SES and BMS) (Table 3, Figure 1).
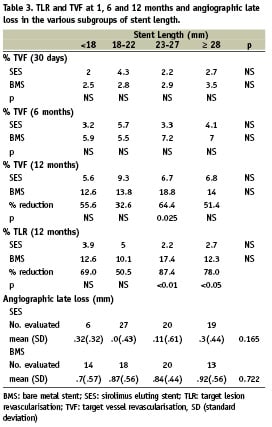
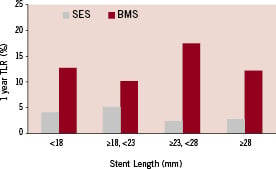
Figure 1. Impact of stent length (as determined by length subgroup) on one year TLR for BMS and SES. It is evident that SES reduces TLR as compared to BMS, however, stent length has no effect on TLR, neither for BMS nor for SES (p=NS for comparisons of the effect of stent length on TLR for BMS and SES, Table 3).
Good quality eight months angiographic data was available in 137 patients. While late loss was smaller in SES as compared to BMS in each of the stent length subgroups there was no length related difference in late loss neither in SES nor in BMS (Table 3, Figure 2).
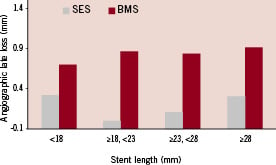
Figure 2. Impact of stent length (as determined by length subgroup) on eight months late loss for BMS and SES. It is evident that SES reduces late loss as compared to BMS, however, stent length has no effect on late loss neither for BMS nor for SES (p=NS for comparisons of the effect of stent length on late loss for BMS and SES, Table 3).
Discussion
An optimal stent for a given lesion should be one that is associated with minimal risk of restenosis and thrombosis. It is well known that long lesions and long stents are associated with a higher risk of restenosis. This relationship is clear with BMS and less pronounced with SES1-4. In an attempt to optimise results, physicians try to match stent to lesion length so that the lesion is fully covered (minimising the risk of edge dissection and thrombosis8), while an attempt is made to use as short a stent as possible to minimise restenosis. In the era of drug eluting stents, physicians tend to use longer stents as compared to BMS, assuming that the impact of stent length on restenosis is much smaller using these stents.
Most of the data regarding the optimal stenting strategy (for BMS and SES) is from studies which excluded patients who underwent primary PCI for AMI. An assumption is made that stenting strategy in these patients should not differ from the strategy used in those without AMI. Our analysis does not confirm the previously described relation between stent length and restenosis. Specifically, there was no difference in TLR at 12 months between patients with short stents (<18 mm) and very long stents (≥28 mm) despite >20 mm mean difference in length. Similarly, eight months angiographic late loss was not influenced by the length of the stent. While this was an expected finding for the SES group, it was totally unexpected for the BMS group. As a consequence, the concept that SES’s are especially useful when long stents are being used was not true for our patients with AMI; the reduction of TLR was significant and its magnitude was independent of stent length.
What could be the explanation for the lack of relation between stent length and clinical restenosis in our patients? There was no consistent increase of stent diameter with increasing length (Table 2) that would explain these findings. Mauri et al9,10 examined the impact of stent length on restenosis in BMS and in SES. Total stent length was divided into stented lesion length (stent covering the atherosclerotic lesion) and excess stent length (stent covering normal vessel segments). Stented lesion length and excess stent length were associated with absolute increases in percent diameter stenosis, per 10 mm, of 9.1% and 3.6% in the BMS and 3.5% and 2.1% in the SES. Thus the impact of excess BMS length on restenosis is similar to that of stented SES lesion length, and is less than half of the effect of stented BMS lesion length. It is also clear from the above analysis that the best predictor for restenosis in BMS is lesion length, and, since longer stents are used for longer lesions, there is also an obvious association between stent length and restenosis.
While lesion length can be easily defined outside the setting of AMI, it is not so in patients with AMI undergoing primary angioplasty. What is considered “lesion” is the combination of the true atherosclerotic lesion and the additional thrombus which very commonly extends to a normal vessel segment. Thus, since true atherosclerotic lesion length is impossible to define in the majority of patients who undergo primary PCI for AMI, the variations in the length of the stents among patients may be due to variations in the length of normal vessels covered with thrombus. This is not expected to cause a major difference in the rate of TLR in the BMS group and obviously not in the SES group.
Study limitations
This is a post-hoc analysis of a study in which the chosen length of the stent was left to the discretion of the operator. Different lengths were chosen by different operators, who were not blinded to stent type, when using BMS or SES for a similar lesion length. However, the main conclusion from our analysis relates to the impact of length on restenosis, rather than on the comparison between the stent types. As is often the case in post hoc analysis, the study was not powered to provide an answer to the question we have raised, so that the lack of impact of stent length on restenosis might be due to the limited power to detect such difference. However, even with this limited power, we can conclude that increasing BMS length from 13 to 35 mm (mean of group one and four respectively) is associated with less than 11% rate difference in one year TLR (95% confidence interval -10% to 11%). Additionally, when a specific length of stent is chosen, safety (stent thrombosis) and efficacy (restenosis) issues should be considered. The analysis examines only restenosis, since the power is limited to assess the impact of length on safety. Due to power limitations, we also elected not to analyse the combined impact of stent diameter and length.
Clinical implications
When choosing a specific length of stent for a given lesion a clinician takes into account factors related to immediate and long term success. Immediate success is determined by anatomic factors (e.g. tortuosity, calcification, side branches etc.), while long term outcome is the result of the effect of stent length on restenosis (and late thrombosis – mainly in DES). True lesion length in the setting of primary PCI in acute MI is very often difficult to determine, and is usually shorter than what is seen in early angiograms. Thus, in many of these patients, most of the stent length does not cover a “true lesion”. Since excess stent length contributes to restenosis much less than stented lesion length (also in BMS) patients treated for acute MI can tolerate long BMS’s without a major increase in the rate of restenosis. This is especially important in the current era when the role of drug eluting stents in primary PCI is under question11. Thus, the physician confronted with a long thrombotic lesion in the setting of AMI should realise that long BMS might not be associated with a prohibitive restenosis rate, while long DES might expose patients to increased risk of late thrombosis.

