
Abstract
Aims: The MASTER study was designed to compare the performance of a new biodegradable polymer sirolimus-eluting stent (BP-SES) with a bare metal stent (BMS) in patients with ST-segment elevation myocardial infarction (STEMI).
Methods and results: The study was a prospective, randomised (3:1), controlled, single-blind multicentre trial that enrolled 500 STEMI patients within 24 hours of symptom onset during 2013-2015. Three hundred and seventy-five patients were treated with BP-SES and 125 with BMS. One hundred and four (104) randomised patients underwent angiographic follow-up at six months. The primary clinical endpoint was target vessel failure (TVF), defined as cardiac death, MI not clearly attributable to a non-target vessel, or clinically driven target vessel revascularisation (TVR) at 12 months. The primary angiographic endpoint was in-stent late lumen loss (LLL) at six months in the angiographic cohort. The major secondary endpoint for safety was a composite of all-cause death, recurrent MI, unplanned infarct-related artery revascularisation, stroke, definite stent thrombosis (ST) or major bleeding at one month. At 12 months, TVF had occurred in 6.1% of BP-SES and 14.4% of BMS patients (pnon-inferiority=0.0004), mainly driven by a higher rate of repeat revascularisation in BMS patients. The safety endpoint occurred in 3.5% of BP-SES and 7.2% of BMS patients (p=0.127). In-stent LLL demonstrated the superiority (p=0.0125) of BP-SES (0.09±0.43 mm) over BMS (0.79±0.67 mm).
Conclusions: The study showed clinical non-inferiority and angiographic superiority of BP-SES versus a comparator BMS, suggesting that this novel DES may be a potential treatment option in STEMI. Clinical Trials Registration: https://clinicaltrials.gov/NCT02828683
Abbreviations
BMS: bare metal stent
BP-BES: biodegradable polymer biolimus-eluting stent
BP-DES: biodegradable polymer drug-eluting stent
BP-SES: biodegradable polymer sirolimus-eluting stent
CABG: coronary artery bypass graft
CAD: coronary artery disease
Co-Cr: cobalt-chromium
DAPT: dual antiplatelet therapy
DES: drug-eluting stent
DP-DES: durable polymer drug-eluting stent
DP-EES: durable polymer everolimus-eluting stent
IRA: infarct-related artery
LLL: late lumen loss
MLD: minimum lumen diameter
POCE: patient-oriented composite endpoint
pPCI: primary percutaneous coronary intervention
QCA: quantitative coronary angiography
RVD: reference vessel diameter
ST: stent thrombosis
STEMI: ST-segment elevation myocardial infarction
TIA: transient ischaemic attack
TLF: target lesion failure
TLR: target lesion revascularisation
TVF: target vessel failure
TVR: target vessel revascularisation
Introduction
In patients with ST-segment elevation myocardial infarction (STEMI), the use of drug-eluting stents (DES) has been associated with favourable outcomes compared with bare metal stents (BMS)1,2. However, stent platform design and polymer technology seem to impact on the clinical outcomes of different DES, as evidenced by the reduced rates of stent thrombosis (ST) with the newer-generation biodegradable polymer (BP) compared with the first generation of durable polymer (DP) DES3. So far, head-to-head randomised comparisons between newer-generation DP-DES versus BP-DES have revealed no significant differences in clinical outcomes, albeit with an inclusion of a mixed population of patients, with a limited number of STEMI4-9. As STEMI patients have a higher risk of repeat ischaemic events, biodegradable polymer DES may reduce long-term stent-related complications10 while providing antiproliferative effects with drug elution11. Despite the diminishing role of BMS in contemporary practice, their use has been maintained in specific patient subsets, including STEMI12. The study presented here aimed to evaluate the outcomes of a new DES, coated with a sirolimus-eluting biodegradable polymer (BP-SES), designed to resorb within three to four months after implantation, thus potentially improving vessel healing and consequently long-term clinical outcomes8,13 in STEMI patients undergoing primary percutaneous coronary intervention (pPCI).
Methods
STUDY POPULATION
This was a prospective, single-blind, multicentre, randomised and controlled clinical study enrolling 500 STEMI patients at 12 European sites and one site in Brazil. STEMI was defined as chest pain >20 minutes and ST-segment elevation of >1 mm in >2 contiguous leads, or (presumably new) left bundle branch block, or true posterior MI with ST depression of >1 mm in >2 contiguous anterior leads on ECG. Primary PCI was performed within 24 hours of symptom onset. At least one acute infarct-related artery (IRA) had to be identified as the target vessel with one or more coronary artery stenoses in a 2.5-4.0 mm native coronary artery which could be treated with one or multiple stents. Key exclusion criteria are listed in Supplementary Appendix 1.
The study was conducted in compliance with the Declaration of Helsinki and an ethics review committee at each participating site approved the study protocol. Written informed consent was obtained from each patient.
RANDOMISATION
Patients were randomly assigned (3:1) to undergo pPCI with either BP-SES or BMS. Randomisation was performed at each site using a sealed envelope system.
STUDY ENDPOINTS
The primary clinical endpoint was target vessel failure (TVF), defined as cardiac death, MI not clearly attributable to a non-target vessel, or clinically driven target vessel revascularisation (TVR) at 12 months. The primary pre-specified angiographic endpoint was in-stent late lumen loss (LLL) at six months post stent implantation in a subset of 104 patients undergoing angiographic follow-up. The major safety secondary endpoint was the composite of all-cause death, recurrent MI, unplanned IRA revascularisation, stroke, definite ST or major bleeding at one month. Other secondary endpoints included target lesion failure (TLF), defined as the composite of cardiac death, MI not clearly attributable to a non-target vessel and clinically driven target lesion revascularisation (TLR) up to 30 days, 6 months, 12 months and annually thereafter up to 3 years. Stent thrombosis was adjudicated according to the Academic Research Consortium definition. Bleeding endpoints were adjudicated according to the Bleeding Academic Research Consortium definition.
A more detailed description of the study endpoints, treatment procedure, blinding and monitoring process and quantitative coronary angiography can be found in Supplementary Appendix 1.
DEVICE DESCRIPTION
The Kaname® BMS stent (Terumo Corporation, Tokyo, Japan) consists of a thin-strut cobalt-chromium (Co-Cr) L605 mesh tube, with an open-cell design. For a 3.0 mm stent, the metallic surface area (nominal) is 15% with a strut thickness of 80 µm and a crossing profile of 0.041”. The available stent lengths were 9, 12, 15, 18, 24, and 28 mm. The Ultimaster® (Terumo Corporation, Tokyo, Japan) consists of the Kaname stent platform, abluminally coated with poly D,L-lactic acid-polycaprolactone (PDLLA-PCL) as a carrier of the immunosuppressant drug sirolimus (3.9 µg/mm stent length). The purpose of the gradient coating is to reduce potential cracking and delamination of the polymer. The drug release profile allows an initial stronger release immediately following stent implantation. Then the drug is released continuously until the polymer bioabsorption is completed within three to four months. For a stent size of 3.0×15 mm, the median maximum concentration (Cmax) was 36.8 pg/mL (range between 22.9 and 41.5 pg/mL), according to the previously published detailed information on the pharmacokinetic profile14.
SAMPLE SIZE AND STATISTICAL ANALYSIS
Sample size calculation for the primary endpoint (TVF) at 12 months was based on expected TVF rates of 4.3% and 8.7% at 12 months in the BP-SES and BMS arms, respectively1. The non-inferiority margin was set at 3% (absolute %TVF for BP-SES no more than 3% higher than BMS) with a one-sided alpha error of 5% and a power of 90%. This required a total of 492 (123 BMS and 369 BP-SES) patients to be randomised. For the primary angiographic efficacy endpoint (in-stent LLL at six months), the following assumptions were used: LLL in the BP-SES and BMS arms was expected to be 0.04±0.35 and 0.80±0.43 mm, respectively. Based on a superiority margin of 0.40 mm with a two-sided alpha error of 5% and a power of 90%, the sample size required was 80 patients (20 BMS and 60 BP-SES). The expected dropout rate for the angiographic follow-up subgroup was 20%.
The primary statistical analysis was performed on the intention-to-treat population. For the comparison of frequencies and means, χ² statistics or Fisher’s exact test and unpaired t-test (with F-test) or non-parametric test (Mann-Whitney or Kruskal-Wallis test for multiple groups comparison) were used, respectively.
Results
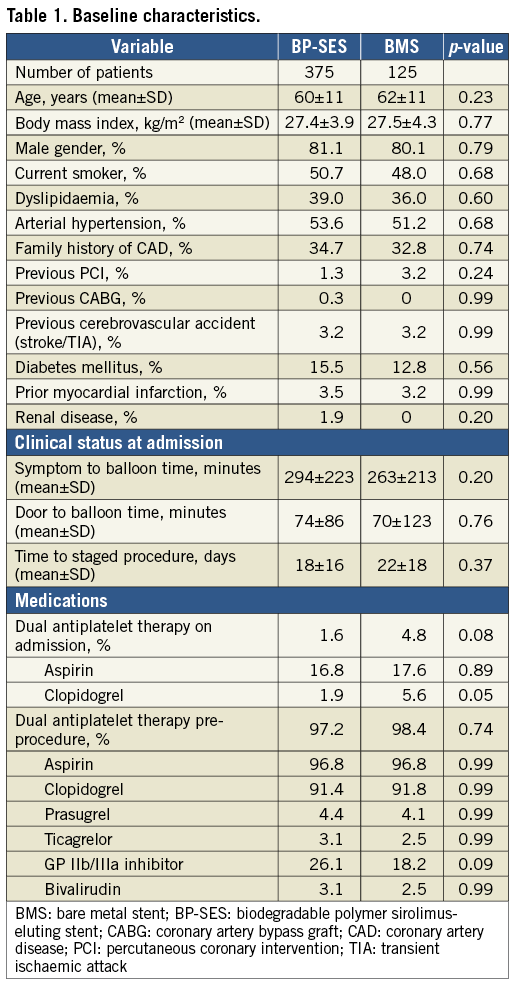
Between October 2013 and March 2015, 500 STEMI patients were enrolled, 375 of whom were assigned to undergo pPCI with implantation of BP-SES and 125 were treated with BMS; 98.4% (n=495) completed the 12-month clinical follow-up (Figure 1). Overall, mean age was 60±11 years, 81% were men and 14.8% of patients had diabetes. There were no significant differences in baseline clinical characteristics between the two study groups (Table 1). Time from symptom onset to first balloon inflation was 286±221 minutes and thrombectomy by manual aspiration was performed in 36% of the overall patient population, with no significant differences between the study groups (Table 1). The DAPT rate did not differ between the groups (Supplementary Table 1).
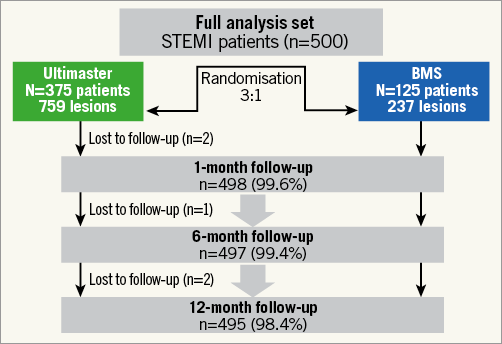
Figure 1. Study flow chart: enrolment, randomisation and follow-up.
While most of the procedural characteristics did not differ significantly between patients receiving BP-SES vs. BMS, the number of implanted stents (1.5±0.9 vs. 1.3±0.6, p=0.039), as well as the mean implanted stent length (30±17 mm vs. 26±12 mm, p=0.012) were significantly greater in the BP-SES group. Staged PCI was performed in 81 patients in the BP-SES group and 16 patients in the BMS group. In total (initial and staged procedure combined), there were more (p=0.003) stents implanted in patients assigned to the BP-SES group (1.8±1.2) versus the BMS group (1.5±0.9) (Supplementary Table 2).
QUANTITATIVE CORONARY ANGIOGRAPHY
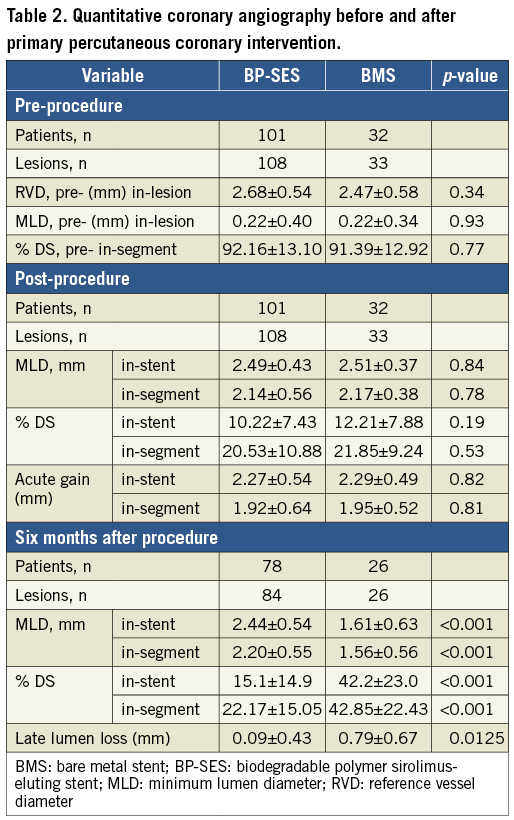
Figure 2 shows the patient flow in the angiographic subset. Pre-intervention measurements showed similar lesion length, minimum lumen diameter (MLD), %DS and reference vessel diameter (RVD) in both groups (Table 2). Post-procedural QCA revealed similar acute luminal gain and residual stenosis after implantation of BP-SES and BMS. At six months, in-stent MLD was larger in BP-SES vs. BMS patients, resulting in significantly lower LLL in BP-SES (0.09±0.43 vs. 0.79±0.67 mm, psuperiority=0.0125), hence meeting the primary angiographic efficacy endpoint of the study (Table 2).
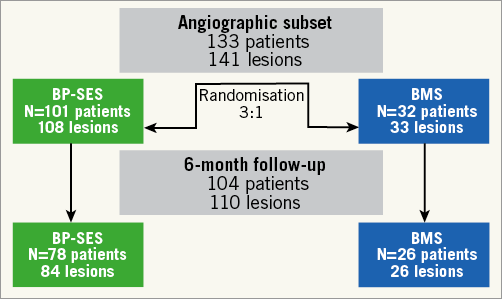
Figure 2. Patient flow in the angiographic subset.
CLINICAL OUTCOMES
At 12 months, BP-SES was non-inferior to BMS with regard to TVF (the primary efficacy endpoint), which occurred in 6.1% vs. 14.4% of patients, respectively, pnon-inferiority=0.0004 (Table 3, Figure 3). This result was predominantly driven by a significant reduction in the rates of TLR/TVR in BP-SES-treated over BMS-treated patients (Table 3).
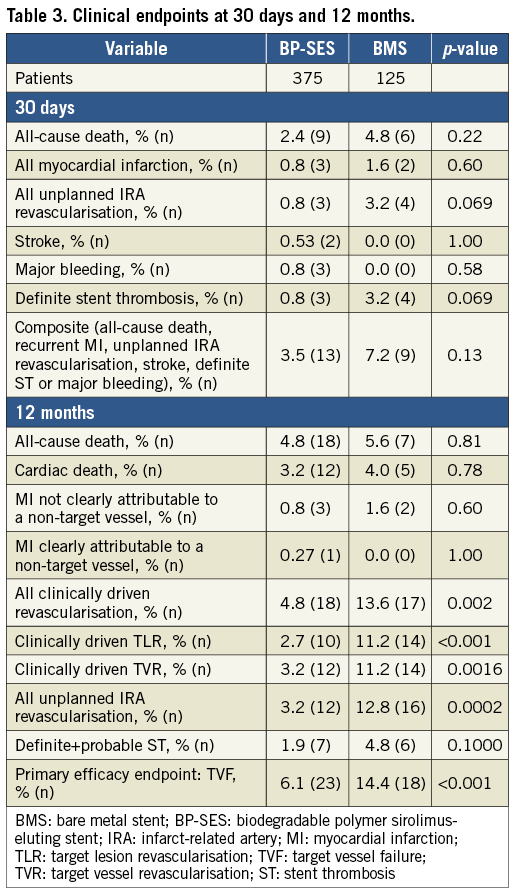
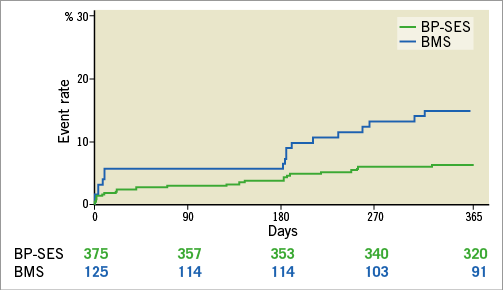
Figure 3. Kaplan-Meier curves of target vessel failure at 12 months.
At 30 days, the composite safety endpoint (all-cause death, recurrent MI, unplanned IRA revascularisation, stroke, definite ST or major bleeding) and the individual components did not differ between the two study groups (Table 3).
Discussion
The main findings of this study comparing a novel BP-SES to a comparator BMS in STEMI patients undergoing pPCI are as follows: 1) this novel DES showed non-inferiority with regard to TVF at 12 months versus BMS, coupled with a reduction in clinically driven repeat revascularisation; 2) in the angiographic subgroup, BP-SES was associated with a significant decrease in late lumen loss compared with BMS, which may explain the significantly lower rate of clinically driven TLR in patients treated with BP-SES; and 3) the cumulative rate of all-cause death, recurrent MI, unplanned IRA revascularisation, stroke, definite ST or major bleeding at one month did not differ between the groups.
The results of recent studies support the hypothesis that a new-generation thin-strut DES with a bioresorbable polymer may be a valuable treatment option for STEMI patients10,15. Although two previous studies have already investigated the effects of newer-generation DES in STEMI patients1,2, to the best of our knowledge this is the first randomised study to compare the results of a thin-strut DES with biodegradable polymer applied abluminally in a gradient fashion, versus a BMS.
The everolimus-eluting stent versus bare metal stent in ST-segment elevation myocardial infarction (EXAMINATION) trial compared a thin-strut (81 µm) DP everolimus-eluting stent (DP-EES) with a BMS. The study did not meet its primary endpoint, defined as a reduction in the patient-oriented composite endpoint (POCE) at one year, which consisted of any death, any reinfarction and any revascularisation2. The rate of TLR up to one year in our study in BP-SES patients (2.7%) was similar to that of DP-EES in the EXAMINATION trial (2.1%), and significantly lower compared to BMS in both trials2.
The effect of a biodegradable polymer DES vs. BMS on cardiovascular events among patients with MI was also tested in the COMFORTABLE AMI trial, that compared a BP biolimus-eluting stent (BP-BES) with 120 µm strut thickness against a corresponding BMS1. The study did meet its primary endpoint as there was a 50% reduction in the combined one-year occurrence of cardiac death, target vessel-related reinfarction, and ischaemia-driven TLR in patients treated with BP-BES. Indeed, the TLR rate in patients treated with BP-BES was significantly lower than that in patients receiving BMS (2.0% vs. 6.2%, p<0.001), and was comparable to the rate found in our BP-SES patients. In our study, the low TLR rate was paralleled by an in-stent LLL of 0.09±0.43 mm at six-month angiographic follow-up. This was lower compared with the previously reported in-stent LLL of 0.19±0.35 mm16 and 0.14±0.36 mm7 after DP-EES implantation, though assessed at a longer (eight to nine months) angiographic follow-up. A pooled analysis of the EXAMINATION and COMFORTABLE AMI trials showed that treatment with DES (DP-EES or BP-BES) was an independent predictor of a lower risk of definite ST (OR 0.35, 95% CI: 0.16-0.74)17. In our study, BP-SES showed a tendency towards lower definite/probable ST rates (Table 3), with most of the observed BMS-related ST occurring in the early post-implantation period (Supplementary Table 3). Although underpowered for this individual endpoint, due to the non-inferiority trial design based on the occurrence of TVF, the tendency to reduce the ST rate may be an indicator of improved prognosis for new-generation DES over BMS in STEMI. As permanent polymer, particularly in first-generation DES, has been associated with impaired vascular healing that may result in increased risk of long-term ST and restenosis18, bioresorbable polymer technology may play a role in minimising the risk of very late ST. However, it should be noted that the observed tendency of BP-SES to reduce the risk of definite/probable ST in the present study was also found in the EXAMINATION trial, indicating that not all permanent polymers are equal in terms of ST risk. In the EXAMINATION trial, the implantation of DP-EES was associated with a significant reduction of definite or probable ST, compared with BMS, at one year (0.9% vs. 2.5%, p=0.02)2. Nevertheless, replacing a permanent polymer layer with a polymer coating that degrades gradually as the drug is released over time (the DES technology tested in the current study)13, thus resulting in a stent surface similar to that of a BMS, has less potential to provide a chronic inflammatory stimulus. The outcomes of the present study seem to support the premise that this type of BP-DES may combine an efficacious antiproliferative effect, as evidenced by the significant TLR and LLL reduction, with an improved safety profile provided by the stent surface and the vessel wall that are free from a polymer. Longer follow-up (five years) will be necessary to confirm this argument further.
The stent thrombosis rate in the present study was slightly higher than in the EXAMINATION and COMFORTABLE AMI studies. However, it is important to note important different aspects between the studies. In the MASTER trial, the studied population was treated with more stents (1.4 vs. 1.1 in the other two studies), longer segments were covered (37 vs. 23 mm), patients were treated less frequently with IIb/IIIa inhibitors (approximately 24% vs. >45%) and less potent antiplatelet regimens were used, compared to the other two trials (mostly clopidogrel and aspirin vs. prasugrel and aspirin). Also, the reported average reference vessel diameter in our study may have been smaller (Table 2) compared to previous studies in STEMI patients. All these things considered, comparisons of the event rates across the studies should be interpreted with caution.
Study limitations
This study has several limitations. First, the study was not powered for individual hard clinical endpoints, and the single-blind design limits robustness. Second, most of the patients were treated with clopidogrel which may not reflect the current standard of care in STEMI patients. Third, a possible imbalance in lesion complexity may have contributed to a higher rate of ST in the BMS group. Fourth, the TLR rate may have, at least in part, been impacted by the six-month angiographic follow-up. However, BMS was associated with a numerically higher rate of TLR also in patients without follow-up angiography (Supplementary Table 4). Fifth, both stroke and major bleeding, as pre-specified parts of the composite safety endpoint, occurred infrequently (together only five events, all in the BP-SES group). When stroke and major bleeding are excluded, BP-SES is associated with a statistically lower rate of the composite of all-cause death, recurrent MI, unplanned IRA revascularisation, or definite ST, as compared with BMS (2.9% vs. 7.2%, respectively, p=0.04). Sixth, despite randomisation, there was an imbalance regarding the staged procedures, in favour of the BP-SES group. Seventh, the protocol allowed the use of non-study stents in staged procedures, so that four patients assigned to a Kaname BMS at index procedure received DES during staged procedures and two patients assigned to an Ulitmaster DES received either BMS or other DES during staged procedures. Importantly, none of these patients had ischaemic events in the follow-up. Finally, longer-term follow-up should be awaited to rule out very late risks with the novel DES technology presented here.
Conclusions
New-generation thin-strut BP-SES exhibited a comparable safety profile to BMS with identical design up to one-year follow-up in STEMI patients. Moreover, lower LLL translated into significantly reduced TVF at 12 months, driven mainly by the TLR reduction. Together, these findings may suggest BP-SES to be another option in percutaneous treatment of patients with STEMI.
| Impact on daily practice Previous research has shown the potential of new-generation DES to improve outcomes over BMS in STEMI patients. Novel technology, including biodegradable polymer DES, may lead to further improvements in the outcomes of patients with STEMI. The MASTER randomised study demonstrated favourable clinical and angiographic results of BP-SES versus a comparator BMS up to 12-month follow-up, indicating that this novel DES may be another treatment option in STEMI. |
Funding
The MASTER clinical trial was funded by Terumo Europe.
Conflict of interest statement
H. Garcia-Garcia declares consultancy fees from Terumo (minor). C. Tamburino reports personal fees from Abbott, personal fees from Medtronic, Stentys, Symetis, and St. Jude, outside the submitted work. M. Zivkovic reports other from Clinical Center Nis during the conduct of the study and is a clinical investigator for the MASTER trial. The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Appendix 1. Methods.
Supplementary Table 1. The rates of dual antiplatelet therapy in patients treated with BP-SES vs. BMS.
Supplementary Table 2. Procedure and lesion characteristics.
Supplementary Table 3. Occurrence of stent thrombosis.
Supplementary Table 4. The rates of TLR in patients treated with BP-SES vs. BMS, in the subgroup of patients with and without angiographic follow-up at six months.
To read the full content of this article, please download the PDF.

