Abstract
Aims: The aim of the study was to clarify the angiographic characteristics of stent thrombosis (ST) in relation to sirolimus-eluting stents (SES).
Methods and results: RESTART is a Japanese registry of SES-associated ST. As an angiographic substudy, coronary angiograms at baseline, at six to 12 months and at the time of ST were analysed. Angiograms of 313 patients (early ST [EST] 169 patients, late ST [LST] 59 patients, and very late ST [VLST] 85 patients) were investigated. Residual dissection post procedure was more frequently seen in the EST group. Stent fracture was more frequently seen in the VLST group than in the EST and LST groups (16.5%, 3.0%, and 3.4%, respectively; p<0.001). Peri-stent contrast staining (PSS), defined as contrast staining outside the stent contour extending to ≥20% of the stent diameter, was remarkably more prevalent in the VLST group than in the EST and LST groups (34.1%, 4.7%, and 6.8%, respectively; p<0.001).
Conclusions: Abnormal angiographic findings such as PSS and stent fracture were found significantly more frequently in lesions with VLST than in lesions with EST and LST.
Introduction
Stent thrombosis (ST) is one of the most serious complications of percutaneous coronary intervention (PCI). It has been reported that very late ST (VLST) beyond one year after stent implantation occurred more frequently after drug-eluting stent (DES) implantation than after bare metal stent (BMS) implantation1. VLST has been reported as continuing to occur until three to four years of follow-up without any signs of attenuation of its incidence2-5. Mechanisms of VLST are currently very poorly understood, and even clinical and angiographic features of VLST after DES implantation have not yet been adequately described, mainly due to its low incidence and emergent situation. We have reported the differences in baseline demographic features, TIMI flow grade and mortality rate, according to the timing of ST in the RESTART registry (REgistry of Stent Thrombosis for review And Re-evaluaTion)6. In addition, we previously demonstrated that peri-stent contrast staining (PSS) found within 12 months after sirolimus-eluting stent (SES) implantation seemed to be associated with subsequent VLST7. However, in the previous study, the prevalence of PSS in patients with VLST was not clarified due to the very small number of definite ST cases in patients with prior PSS diagnosis, and the lack of detailed angiographic analysis at the time of VLST.
In the current angiographic substudy of the RESTART registry, we sought to evaluate the detailed angiographic findings at the time of SES implantation, at six to 12 months follow-up, and at the time of ST. Qualitative and quantitative angiographic parameters were compared according to the timing of ST.
Methods and design
STUDY POPULATION
The RESTART registry is a nationwide Japanese registry of patients with ST after SES implantation. Design and patient enrolment of the RESTART registry have been published previously6. In the current angiographic substudy, coronary angiograms at baseline and at the time of ST were analysed in an angiographic core laboratory (Cardiocore, Tokyo, Japan). In cases of VLST, scheduled follow-up angiograms performed at six to 12 months were also analysed. The study protocol was approved by the ethics committees of the two centres to which the two principal investigators are affiliated (Kyoto University Hospital and Teikyo University Hospital). Because this study was conducted retrospectively, written informed consent was waived according to the guidelines for epidemiologic studies issued by the Ministry of Health, Labour and Welfare of Japan.
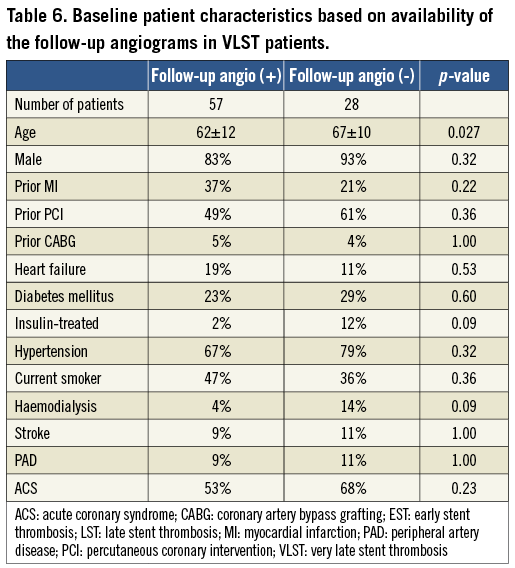
Among 235 centres reporting ST cases in the RESTART registry, 154 centres agreed to participate in the angiographic substudy (Online Appendix). The study population of the angiographic substudy consisted of 313 patients with ST out of the entire cohort of 611 patients enrolled in the RESTART registry. All angiograms with ST from centres participating in the angiographic substudy were analysed. There were 169 patients with early ST (EST), 59 patients with late ST (LST), and 85 patients with VLST (Figure 1). Angiograms both at baseline procedure and at the time of ST were analysed in all cases. Follow-up angiograms at six to 12 months were performed based on the sites’ discretion and analysed in 57 cases (67%) out of 85 cases with VLST.
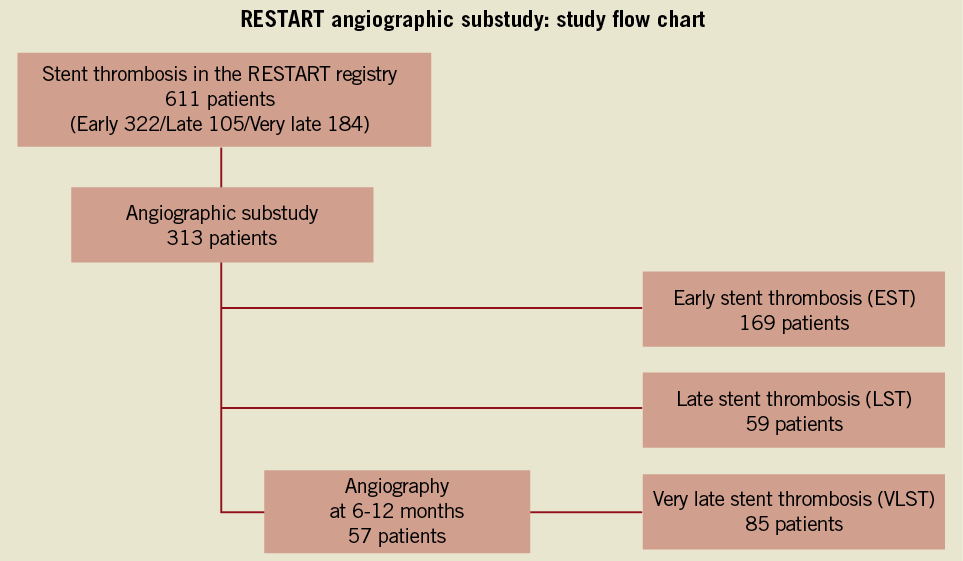
Figure 1. Study flow chart for the angiographic substudy of the RESTART registry. RESTART: registry of stent thrombosis for review and re-evaluation; CAG: coronary angiography; EST: early stent thrombosis; LST: late stent thrombosis; VLST: very late stent thrombosis
DEFINITIONS
ST was defined according to the Academic Research Consortium (ARC) definition8. Only patients with ARC definite ST were enrolled in the RESTART registry. ST was judged by the site investigators to have occurred if thrombolysis in myocardial infarction (TIMI) flow was grade 0 or 1 with occlusion originating in the peri-stent region, or grade 2 or 3 in the presence of angiographic evidence of thrombus associated with signs and symptoms of acute coronary syndrome (ACS). ST was categorised according to the timing of the occurrence of ST into EST (within 30 days), LST (between 31 and 365 days) and VLST (beyond one year).
ANGIOGRAPHIC ANALYSIS
Each angiogram was assessed both qualitatively and quantitatively by two experienced interventional cardiologists in an independent angiographic core laboratory (Cardiocore, Tokyo, Japan). Quantitative coronary angiographic analysis (QCA) was carried out with the CAAS 5.4 (Pie Medical Imaging, Maastricht, The Netherlands). The target segment was defined as the entire segment involving the implanted stent and the 5 mm proximal and distal edges adjacent to the stent. Angiographic analysis at the time of ST included angiographic evidence of thrombus, TIMI flow grade, stent fracture and PSS. Stent fracture was detected on coronary angiography according to the previously reported definition9, which was either partial strut fracture or complete separation of the stented segment. At the time of ST, PSS was evaluated after thrombus aspiration and/or balloon angioplasty. PSS was defined by discussion among six investigators (Kimura, Kadota, Imai, Tada, Suzuki and Kozuma) as contrast staining outside the stent contour extending to ≥20% of the stent diameter, considering the resolution of QCA (3 pixels exceeding the stent border from the 3.0 mm stent), as previously described7. Timing of PSS was classified as resolved PSS (PSS observed at post-stenting but not at follow-up), persistent PSS (PSS observed both at post-stenting and follow-up) and late acquired PSS (PSS observed at follow-up but not at post-stenting). In the current analysis, only late acquired PSS was evaluated. Angiograms of typical PSS and subsequent ST are demonstrated in Figure 2. The extent of PSS was measured using the caliper method of QCA software to determine whether the depth of the contrast staining exceeded 20% of the stent diameter. When a discrepancy between the two observers was found, the final decision was made by discussion and consensus in the angiographic core laboratory. In 98% of the cases (306 out of 313 lesions), the two observers agreed on the presence or absence of PSS.
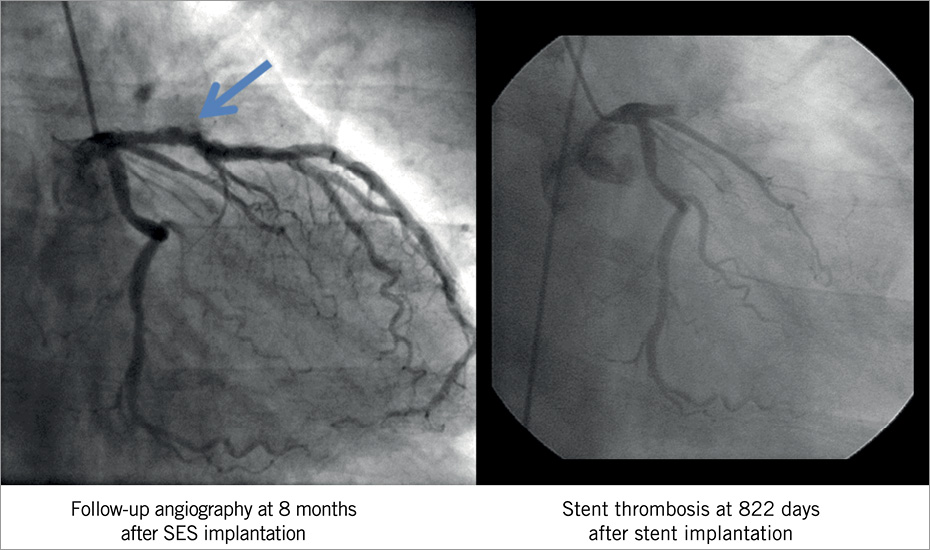
Figure 2. A representative case of PSS diagnosed at 8-month angiographic follow-up with subsequent VLST at 822 days after SES implantation. Arrow indicates the area of PSS. PSS could not be identified at the time of VLST. PSS: peri-stent contrast staining; SES: sirolimus-eluting stent; VLST: very late stent thrombosis
IVUS ANALYSIS
IVUS data of VLST cases have been collected to clarify the mechanism of PSS findings, retrospectively. Maximum vessel area and the remodelling index were measured by means of PC-based software (EchoPlaque; Indec Systems Inc., Santa Clara, CA, USA). Incomplete stent apposition (ISA) was evaluated according to the previous definition10. IVUS analysis was conducted in an independent core laboratory at Stanford University Medical Center (Cardiovascular Core Analysis Laboratory, Stanford, CA, USA).
STATISTICAL ANALYSIS
Categorical variables were compared with the chi-square test. Continuous variables were expressed as mean value ± standard deviation, unless otherwise indicated. Continuous variables were compared using the analysis of variance (ANOVA).
All analyses were performed with the use of SPSS software version 16 (SPSS Inc., Chicago, IL, USA) and all reported p-values were two-sided. A p-value of <0.05 was regarded as statistically significant.
Results
BASELINE CLINICAL CHARACTERISTICS
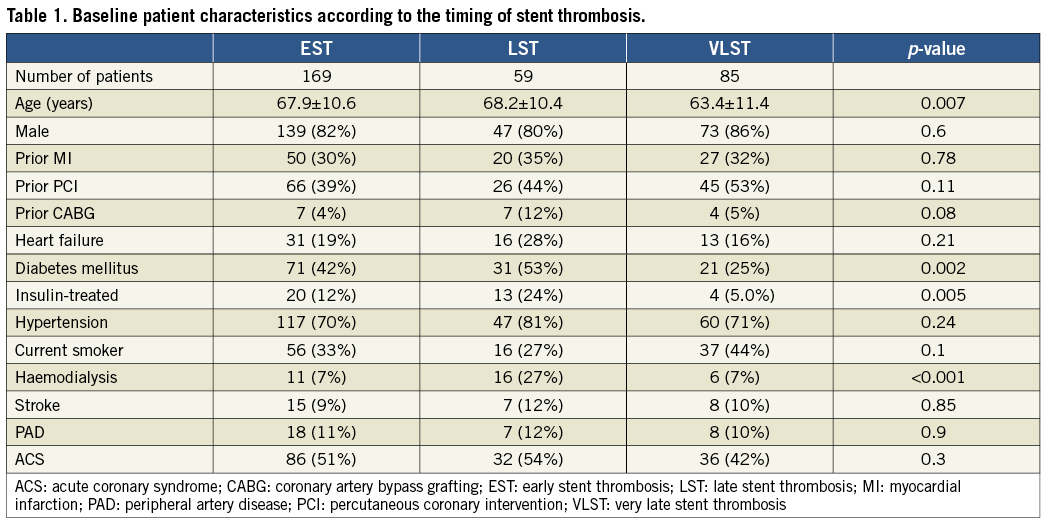
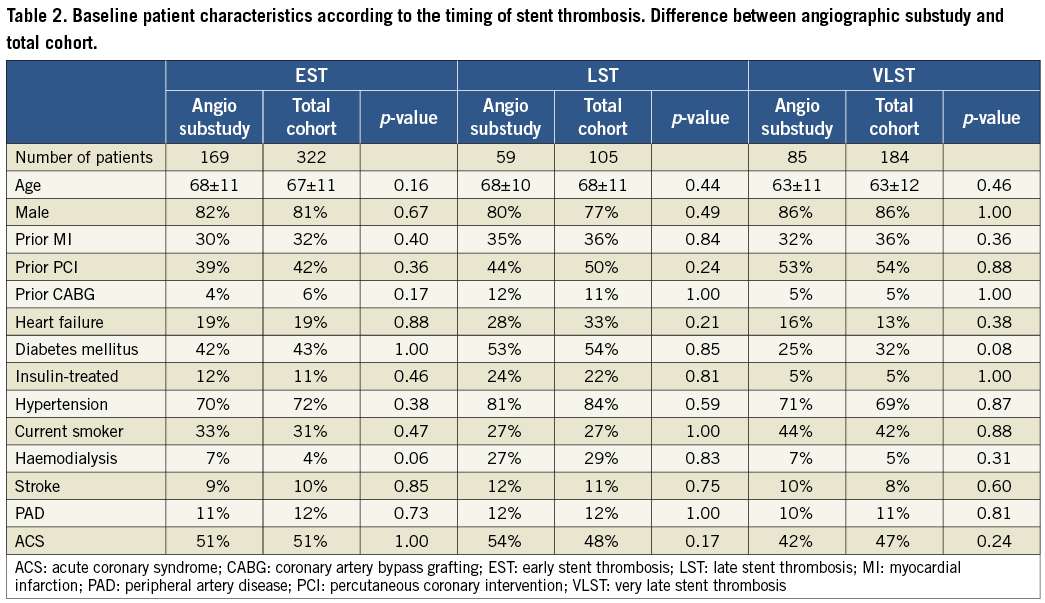
Baseline patient characteristics were significantly different according to the timing of ST, which was consistent with the results in the entire cohort of the RESTART registry (Table 1 and Table 2)6. In brief, patients in the VLST group were younger and were less often diabetics. Patients on haemodialysis and patients with diabetes were more prevalent in the LST group.
ANGIOGRAPHIC FINDINGS AT BASELINE
Angiographic findings at baseline were also different in several aspects. Residual dissection post procedure was more frequently seen in the EST group. Chronic total occlusion was more prevalent and severe calcification was less prevalent in the VLST group. The prevalence of well-known risk factors of ST such as bifurcations and overlapping long stents was high and was not different according to the timing of ST (Table 3). Minimal lumen diameter post stenting was significantly greater and percent diameter stenosis post stenting was significantly smaller in the VLST group (Table 4).
ANGIOGRAPHIC FINDINGS AT THE TIME OF STENT THROMBOSIS AND AT SIX TO 12 MONTHS AFTER SES IMPLANTATION
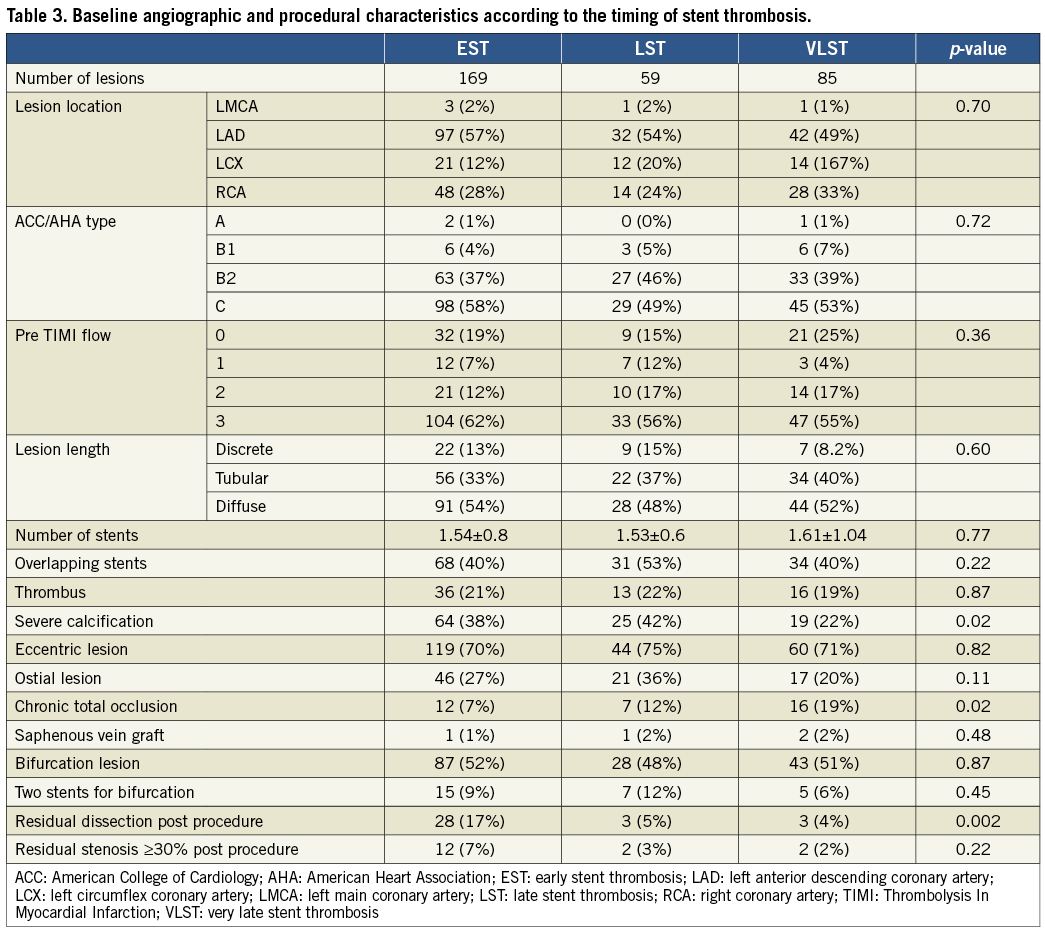
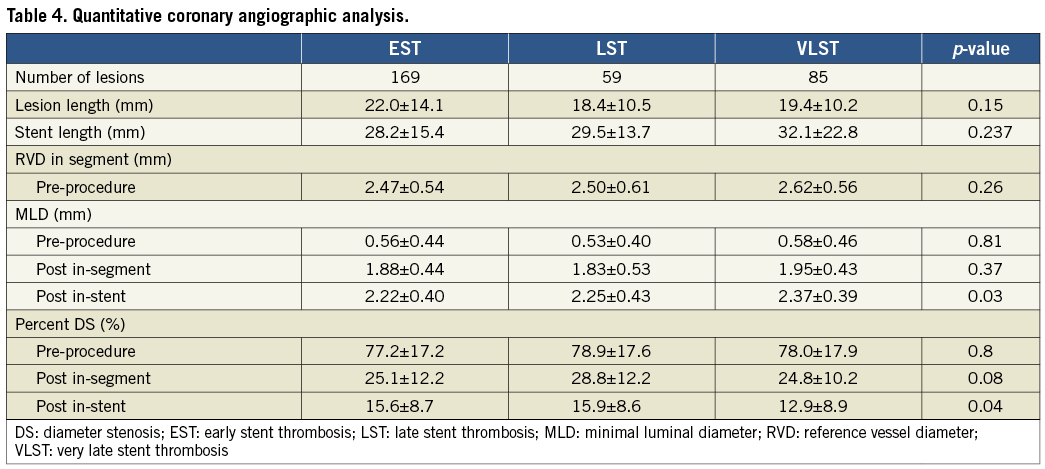
At the time of ST, angiographic evidence of thrombus was confirmed in 301 lesions (96.2%). TIMI 2/3 flow was observed more frequently in the LST group (Table 5). Stent fracture was more frequently seen in the VLST group than in the EST and LST groups (16.5%, 3.0%, and 3.4%, respectively; p=0.050). PSS was remarkably more prevalent in the VLST group than in the EST and LST groups (34.1%, 4.7%, and 6.8%, respectively; p<0.001) (Figure 3). A case of PSS at VLST is shown in Figure 4. Mean depth of PSS measured by QCA was 0.87±0.30 mm. The extent of PSS was 3.13±1.54 mm in length. Among 29 PSS cases, four lesions (13.8%) were aneurysms (>1.5 times of reference diameter). In lesions with PSS at the time of VLST, PSS had already been demonstrated in 14 lesions (56%) at six to 12 months angiographic follow-up. Even in lesions without PSS at the time of VLST, PSS had also been observed in 13 lesions (41%) at six to 12 months angiographic follow-up. Therefore, PSS was documented at least once during follow-up in 42 lesions (69% of 61 lesions with six to 12 months angiographic follow-up and 49% of 85 lesions with VLST) (Figure 5). Persistent PSS was observed in one patient with VLST and in three patients with early and late ST. Dual antiplatelet therapy was maintained at the time of ST in only five out of 27 VLST patients with the finding of PSS (18.5%), and in four out of 14 VLST patients (28.6%) showing stent fracture.
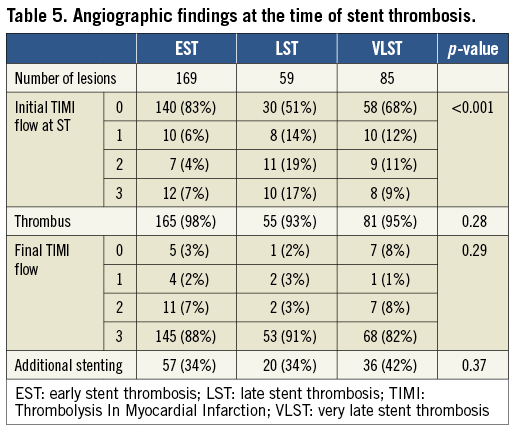
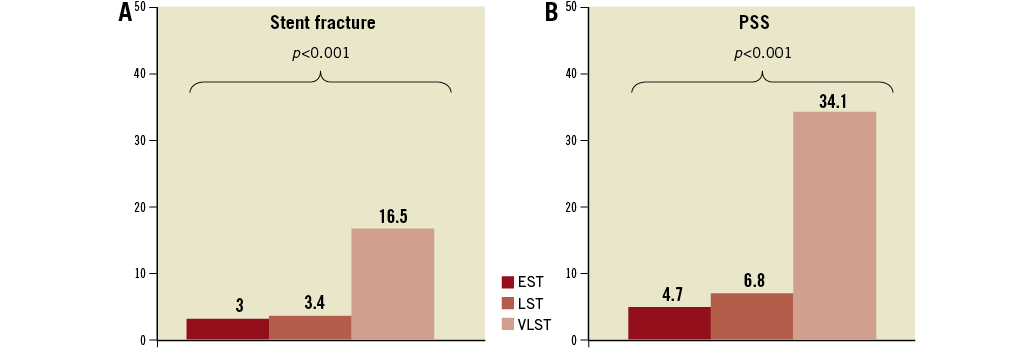
Figure 3. Prevalence of stent fracture (A) and PSS (B) according to the timing of ST. EST: early stent thrombosis; LST: late stent thrombosis; PSS: peri-stent contrast staining; ST: stent thrombosis; VLST: very late stent thrombosis
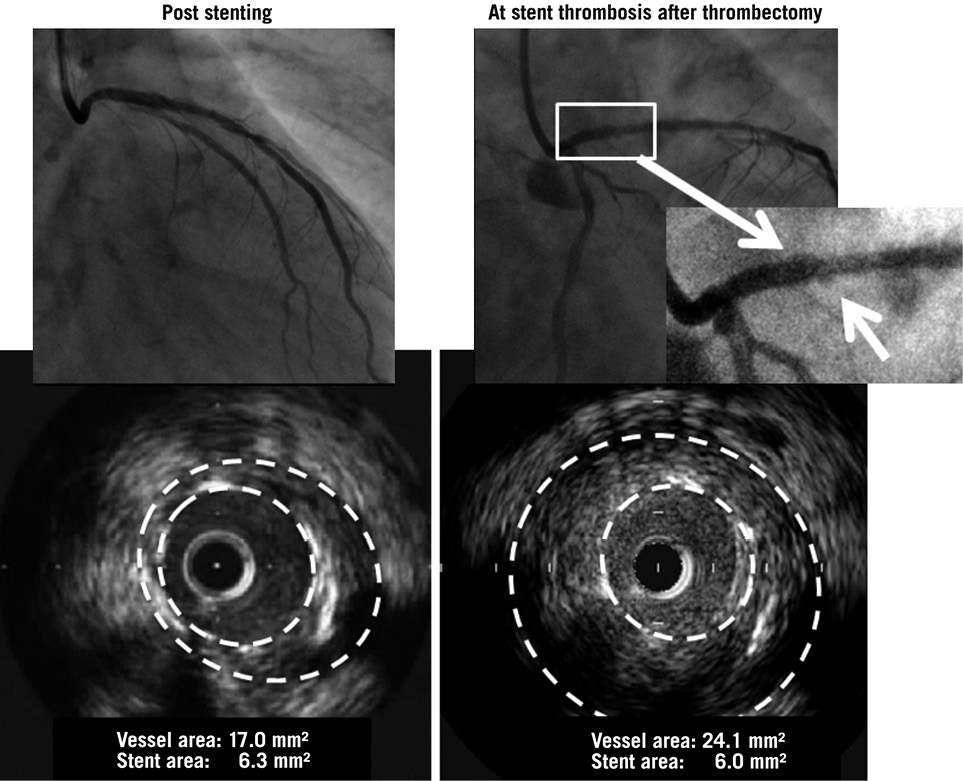
Figure 4. A representative case of very late stent thrombosis with diagnosis of PSS at the time of stent thrombosis (arrow). PSS was not identified post procedure. Maximum vessel area was enlarged from 17.0 mm2 to 24.1 mm2 and the remodelling index increased from 1.13 to 1.60 by IVUS analysis. PSS: peri-stent contrast staining; SES: sirolimus-eluting stent
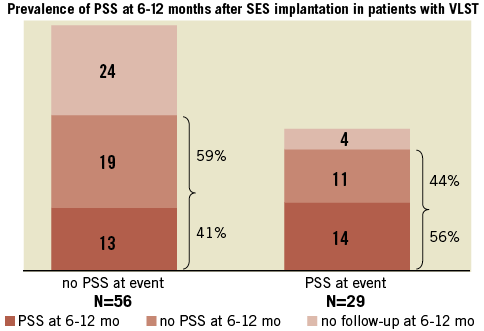
Figure 5. Prevalence of PSS at 6-12 months follow-up angiography in lesions with and without PSS at the time of VLST. PSS had already been diagnosed in 56% of lesions with PSS at event and in 41% of lesions without PSS at event. PSS: peri-stent contrast staining; VLST: very late stent thrombosis
CORRELATION WITH IVUS ANALYSIS
IVUS data at VLST were available in 14 patients among 85 patients with VLST. Of these, four cases presented PSS in angiography, which was confirmed by IVUS as ISA in three cases (75%). Maximum vessel area was 31.2±12.2 mm2 in patients with PSS and 23.4±5.8 mm2 in patients without PSS, although serial data were obtained in only one case. Maximum vessel area of PSS cases was 24.11 mm2, 20.34 mm2, 47.84 mm2, and 32.32 mm2, respectively (Figure 4). Regarding the non-PSS cases at VLST (n=10), ISA was observed in six cases (60%).
Discussion
The main findings of this study are the following: (1) abnormal angiographic findings named PSS were found frequently at the time of VLST, but not at the time of EST and LST; (2) stent fracture was also significantly more prevalent in lesions with VLST than in lesions with EST and LST.
In several intravascular ultrasound (IVUS) studies, late acquired ISA with positive remodelling was reported to be seen in 5-13% of lesions treated with SES, but was rarely seen in lesions treated with BMS10-12. More recently, an association between late acquired ISA and VLST has been suggested by several IVUS studies demonstrating a very high prevalence (73-77%) of ISA in the setting of VLST13-16. PSS might be an angiographic counterpart of ISA observed by IVUS. The observed prevalence of PSS (34%) at the time of VLST was lower than the reported prevalence of ISA by IVUS (73-77%) reflecting the higher sensitivity of IVUS in detecting ISA. Difficulty in detecting PSS at the time of ST due to thrombotic material filling the space behind the stents could also explain the different prevalence of ISA or PSS between angiography and IVUS. Actually, even in lesions without PSS at the time of VLST, PSS had been observed in 41% of lesions at six to 12 months angiographic follow-up.
Although IVUS demonstrated higher sensitivity in detecting ISA, it is not realistic to perform a follow-up IVUS examination in order to identify incomplete apposition and stent fracture in routine clinical practice. Routine angiographic follow-up at six to 12 months after stent implantation is still commonly performed in Japan, even in the DES era. Considering the very low incidence of VLST, it seemed to be an inefficient way to conduct systematic angiographic follow-up to detect PSS and stent fracture. However, if PSS and/or severe stent fracture could predict future VLST, detection of these abnormal angiographic findings would be clinically relevant, although we do not recommend systematic scheduled follow-up angiography. We have previously demonstrated that PSS found within 12 months after SES implantation seemed to be associated with subsequent VLST7. Therefore, when PSS is observed at clinically-driven follow-up angiography, meticulous care including prolonged dual antiplatelet therapy may be required to prevent the occurrence of ST, considering the fact that dual antiplatelet therapy is stopped in the majority of VLST patients with a PSS finding.
It is intriguing that stent fracture was also significantly more prevalent in lesions with VLST than in lesions with EST and LST, although stent fracture is usually caused by fatigue of the devices. In the current study, the prevalence of stent fracture in lesions with VLST was much higher than the previously reported incidence (1-3%) during follow-up after DES implantation17. A human autopsy study in 177 DES-treated lesions reported a very high incidence of stent fracture (29%) and adverse pathologic reaction such as thrombosis and/or inflammation associated with severe stent fracture18. In an IVUS study of 20 consecutive lesions with IVUS evidence of stent fracture, stent fracture was reported to be associated with coronary aneurysm in five lesions (all treated with SES)19. It was postulated that biological reactions to the drug-eluting stent may cause positive remodelling with aneurysm formation and malapposition; aneurysm formation and malapposition may allow motion and/or kinking of the stent within the aneurysm, leading to stent fracture. Therefore, stent fracture seemed not to be the cause of PSS, but the consequence of PSS.
Delayed arterial healing has been considered to be the major cause of DES thrombosis20. However, patient characteristics and angiographic findings at the time of ST were markedly different according to the timing of ST in the current study. Suspected mechanisms for EST included procedural factors such as residual dissection, platelet activation, and thrombotic milieu present in acute myocardial infarction. Differences in endothelial regeneration between DES and BMS might be a cause of LST20. However, the high prevalence of PSS and ISA with positive remodelling in lesions with VLST might suggest that major mechanisms of VLST are different from delayed endothelial healing, although PSS and ISA with positive remodelling could be a continuous process resulting from delayed healing. Cook et al evaluated 10 patients with VLST by histologic examination of the thrombi aspirated, demonstrating that VLST was associated with inflammation14. They also demonstrated that eosinophilic infiltrates were more common in thrombi harvested from patients with VLST as compared with other causes of myocardial infarction, suggesting the presence of hypersensitivity in these lesions with VLST of DES. Virmani et al reported a patient with VLST of SES, demonstrating histopathologic findings of extensive inflammation in the intima, media, and adventitia, consisting predominantly of lymphocytes and eosinophils associated with aneurysmal dilatation of the vessel wall, malapposition of the stent struts and thick fibrin thrombus between the stent struts and arterial wall21,22. More recently, Kon et al reported a case with VLST of SES demonstrating serial changes in contrast staining outside the stent border leading to aneurysm formation as well as histopathologic evidence of hypersensitivity vasculitis in the stented segment23. Therefore, accumulating evidence suggests that chronic inflammation may play the cardinal role in the pathogenesis of VLST. If hypersensitivity reaction and inflammation are the main cause of VLST, it may be reasonable that younger patients with less calcified lesions are dominant in VLST cases.
Limitations
There are several important limitations in the current study. First and most importantly, although the current angiographic analysis included the largest number of patients with SES-associated ST, we did not have data on control patients without ST. Therefore, we could not evaluate the risk factors of ST according to the timing after SES implantation. Second, there is a possibility of selection bias because only half of the patients in the RESTART registry were enrolled for the angiographic substudy. Furthermore, we have to admit that we could not guarantee all consecutive enrolments of ST patients in the RESTART registry by all centres, because case submission was conducted by the voluntary efforts of the sites. Third, six to 12-month angiograms were analysed only in 67% of VLST cases (Table 6). In addition, routine angiographic follow-up, which is a common practice in Japan, may have influenced the outcome since investigators may have altered their treatment according to the angiographic findings. However, the majority of hospitals performed routine angiographic follow-up after drug-eluting stent implantation regardless of initial angiographic results and recurrent ischaemic signs. Fourth, stent fracture could easily have been missed due to insufficient resolution of QCA, particularly when the stent was not heavily disrupted or image quality was not sufficient. In addition, assessment of PSS after balloon dilatation was challenging, since balloon dilatation sometimes induces dissection outside the stent. Fifth, IVUS analysis was not intended to be included in this angiographic substudy. Therefore, collection of IVUS images was insufficient and IVUS analysis may have less relevance in the current study. Finally, only a large-scale longitudinal follow-up study in patients with PSS and/or stent fracture could address the real clinical impact of these abnormal angiographic findings after SES implantation.
Conclusions
Abnormal angiographic findings such as PSS and stent fracture were found significantly more frequently in lesions with VLST than in lesions with EST and LST. The underlying mechanisms of VLST might be different from those of EST and LST.
Acknowledgements
We appreciate the efforts of the investigators in the 154 participating centres and of the clinical research coordinators supporting the study (see supplementary file). We also thank Dr Kazushige Kadota in Kurashiki Central Hospital for his support in defining and classifying PSS findings. We are especially indebted to Ms Emi Hiyoshi and Mr Eizo Nishimura (Cordis Cardiology, Japan; Johnson & Johnson, Tokyo, Japan) for the designing and construction of the study.
Funding
Cordis Cardiology, Japan; Johnson & Johnson, Tokyo, Japan. The study sponsor was involved in the discussion of the study design and in the collection of data. However, the study sponsor was not involved in the analysis and interpretation of data, in the writing of the report, and in the decision to submit the paper for publication.
Conflict of interest statement
The following relationships with the sponsors of the study (Cordis Cardiology, Japan; Johnson & Johnson, Tokyo, Japan) were reported by the authors: K. Kozuma: receipt of honoraria; T. Kimura; advisory board member, speaker, and recipient of research grants; T. Aizawa: advisory board member, receipt of honoraria; S. Miyazaki: advisory board member; T. Yamaguchi: advisory board member; T. Isshiki: advisory board member, receipt of honoraria. The other authors reported no conflicts of interest.
Appendix. List of the participating centres and investigators of the RESTART registry angiographic substudy.
Asahikawa Medical College Hospital: Naoyuki Hasebe, Toshiharu Takeuchi; Teine Keijinkai Hospital: Mitsugu Hirokami, Yoshikazu Asano; Cardio-vascular Center Hokkaido Ohno Hospital: Takehiro Yamashita, Toshiya Satoh; Caress Sappro Hokko Memorial Hospital: Masayuki Sakurai, Yoichi Nozaki; Hirosaki University Hospital: Ken Okumura, Takumi Higuma; Hachinohe City Hospital: Yoichi Inokubo; Iwate Medical University Hospital: Tomonori Itoh; Iwate Prefectural Central Hospital: Akihiro Nakamura; Miyagi Cardiovascular & Respiratory Center: Yoshiaki Mibiki; Sendai Cardiovascular Center: Masahiro Yagi, Shinya Fujii; Akita University Hospital: Hiroshi Ito, Kenji Iino; Sanyudo Hospital: Hideki Abe, Osamu Kawashima; Takeda General Hospital: Hiroshi Seida; Ohta Nishinouti Hospital: Nobuo Komatsu; Hoshi General Hospital: Mikihiro Kijima, Yuichi Ujiie; Tsuchiura Kyodo General Hospital: Tunekazu Kakuta, Taro Iwamoto; Ibaraki Prefectural Central Hospital: Noriyuki Takeyasu, Daisuke Abe; Ushiku Aiwa General Hospital: Masahiro Abe; Ryugasaki Saiseikai Hospital: Masahiro Toyama; Tsukuba Medical Center Hospital: Yuichi Noguchi, Hidetaka Nishina; Jichi Medical
University Hospital: Takaaki Katsuki, Tomokazu Ikemoto; Public Fujioka General Hospital: Masahiro Inoue; Motojima General Hospital: Teiji Motojisma, Shuhei Kubota; Saitama Medical Center Jichi Medical University: Norifumi Kubo, Hiroshi Funayama; Fukaya Red Cross Hospital: Masao Yamazaki, Makoto Sekiguchi; Chiba University Hospital: Yoshio Kobayashi, Naoki Ishio; The Jikei University School of Medicine Kashiwa Hospital: Mitsuyuki Shimizu, Toshio Hasuda; Chibanishi-General Hospital: Kazuo Misumi; Chiba Aoba Municipal Hospital: Nobuaki Shikama; Nippon Medical School Hospital: Kyoichi Mizuno, Hitoshi Takano; Tokyo Medical University Hachioji Medical Center: Kenji Takazawa, Hiroshi Kobayashi, Mineko Kinou; Kanto Medical Center NTT Ec: Satoshi Ohnishi, Masao Yamasaki; Tokyo Kosei Nenkin Hospital: Seiji Ayabe; Mitsui Memorial Hospital: Kazuhiro Hara, Jiro Aoki; Kawakita General Hospital: Yoichi Sugimura, Akio Oshima; Ome Municipal General Hospital: Kenichiro Otomo, Shigeo Shimizu; Shinkatsushika Hospital: Youichi Shimizu, Masayoshi Sakakibara; Sakakibara Heart Institute: Tetsuya Sumiyoshi, Ryuta Asano; Tokai University Hachioji Hospital: Takahiko Kiyooka; Showa University Northern Yokohama Hospital: Masahiko Ochiai, Kazuhiro Ashida, Tadayuki Yakushiji; St. Marianna University School of Medicine: Haruki Musha, Masahiro Yamauchi; Tokai University Hospital: Yoshihiro Morino, Toshiharu Fujii; Yokohama City University Medical Center: Kazuo Kimura, Jun Okuda; Yokohama Sakae Kyosai Hospital: Ichiro Michishita; Hiratsuka Kyosai Hospital: Shigeo Umezawa, Yuko Onishi; Tomei-Atsugi Hospital: Fumihiko Usuba, Takehiko Nakamura; Niigata City General Hospital: Hirotaka Oda, Keiichi Tsuchida; Tachikawa General Hospital: Masaaki Okabe, Minoru Takahashi; Niigata Kobari Hospital: Hideaki Otsuka, Kotaro Higuchi; Toyama Prefectural Central Hospital: Kazuo Usuda, Yoshiki Nagata; Kofu Kyoritsu Hospital: Yoko Kurumatani; Nagano Red Cross Hospital: Jiro Yoshioka; Aizawa Hospital: Syunpei Sakurai, Tomohiro Suzuki; Nakatsugawa Municipal General Hospital: Kazunori Hayashi; Shizuoka General Hospital: Osamu Doi, Satoshi Kaburagi; Seirei Mikatahara General Hospital: Yasushi Wakabayashi, Makoto Sano; Shimada Municipal Hospital: Takeshi Aoyama, Michitomo Kawahito; Kakegawa City General Hospital: Hirohide Uchiyama, Hirohumi Okazaki; Hamamatsu Medical Center: Masakazu Kobayashi; Fujinomiya City General Hospital: Nobuyuki Wakahara; Nagoya University Hospital: Hideki Ishii; Aichi Medical University Hospital: Takayuki Ito, Hiroaki Takashima; Nagoya Daini Red Cross Hospital: Haruo Hirayama, Mamoru Nanasato; Chukyo Hospital: Naoya Tsubo, Kenji Kada; Nagoya Ekisaikai Hospital: Toshikazu Sofue, Daizou Ishihara; Anjo Kosei Hospital: Kenji Takemoto; Okazaki City Hospital: Toshikazu Tanaka, Satoshi Yanagisawa; Kasugai Municipal Hospital: Akihiro Terasawa, Yuzo Hayashi; Toyohashi Municipal Hospital: Osamu Ohno, Kenshin Naruse; Meijo Hospital: Yoshio Iwama, Akira Kimura; Komaki City Hospital: Katsuhiro Kawaguchi; Toyohashi Heart Center: Takahiko Suzuki, Masashi Kimura; Suzuka General Hospital: Takuya Mori, Tetsuya Seko; Mie Heart Center: Hideo Nishikawa, Hiroyuki Suzuki; Koto Memorial Hospital: Tomoyuki Murakami, Hiroshi Mabuchi; Red Cross Otsu Hospital: Takashi Konishi, Yoshihito Takimoto; Otsu Municipal Hospital: Nobuyuki Tanaka, Yoshikazu Shigemoto; Omihachiman Community Medical Center: Hirotaka Tatsukawa, Daisuke Kanbayashi; Hikone Municipal Hospital: Yoshihiro Himura, Tsuyoshi Miyazawa; Shiga Medical Center for Adults: Shigeru Ikeguchi, Kunihiko Kosuga; Social Insurance Shiga Hospital: Osamu Yamaoka; Kyoto University Hospital: Takeshi Kimura; Red Cross Kyoto Daiichi Hospital: Yoshio Kohno, Masayuki Hyogo; Daini Okamoto General Hospital: Takafumi Yagi; Yamashiro Public Hospital: Kiichiro Tomiyasu, Satoshi Akabame; Shimabara Hospital: Mamoru Takahashi, Yoshiki Matoba; Osaka City University Hospital: Tohru Kataoka, Satoshi Nishimura; Osaka Medical College Hospital: Shuji Suzuki; Kansai Medical University Takii Hospital: Yoshihiro Yamamoto; Osaka Red Cross Hospital: Tsukasa Inada, Fujio Hayashi; Osaka Saiseikai Noe Hospital: Shunsuke Take, Shiho Koyama; Sumitomo Hospital: Hisatoyo Hiraoka, Yuji Yasuga, Masami Miyawaki; Saiseikai Ibaraki Hospital: Takashi Tamura, Mariko Tanaka; Yao General Hospital: Shozo Tanaka; Kishiwada City Hospital: Mitsuo Matsuda, Takashi Uegaito; Nozaki Tokushukai Hospital: Satoru Sumitsuji, Masaaki Okutsu, Kashima Ito; Osaka Ekisaikai Hospital: Kenei Shimada, Haruyuki Taguchi; Kobe City Medical Center General Hospital: Yutaka Furukawa, Natuhiko Ehara; Kansai Rosai Hospital: Shinsuke Nanto, Masaki Awata; Hyogo Prefectural Amagasaki Hospital: Yoshiki Takatsu, Ryoji Taniguchi; Miki City Hospital: Kojiro Awano, Yoshitaka Ohashi; Kobe Red Cross Hospital: Yuichi Kuroda; Akashi Medical Center: Masahito Kawata; Higashi Takarazuka Satoh Hospital: Satoru Otsuji, Masashi Ikushima; Nara Hospital, Kinki University: Manabu Shirotani; Tenri Hospital: Yoshihisa Nakagawa, Jiro Sakamoto; Mimuro Hospital: Naofumi Doi, Yasuhiro Takeda; Japanese Red Cross Society Wakayama Medical Center: Hiroki Sakamoto, Hiroshi Ueda; Tottori University Hospital: Osamu Igawa, Yosuke Furuse; Matsue Red Cross Hospital: Nobuo Shiode, Fumiyo Tunoda; Shimane Prefectural Hospital: Tsuyoshi Oda; Masuda Red Cross Hospital: Tadahiko Minoji, Toshihiko Uchida; Kawasaki Medical School Hospital: Hiroyuki Okura, Takahiro Kawamoto; Okayama Medical Center: Yoshihisa Fujimoto; Kurashiki Central Hospital: Kazuaki Mitsudo, Masao Imai; Hiroshima University Hospital: Yasuki Kihara, Futoshi Tadehara; National Hospital Organization Kure Medical Center: Ritsu Tamura, Hirohiko Nishiyama; Higashihiroshima Medical Center: Kaoru Yanagihara, Yujiro Ono; Fukuyama City Hospital: Makoto Nakahama, Katsushi Hashimoto; Mazda Hospital: Kotaro Sumii, Yuichi Orita; Tsuchiya General Hospital: Yasuhiko Hayashi, Mamoru Toyofuku, Miyo Hatanari; Hiroshima-Nishi Medical Center: Hitoshi Fujiwara, Takashi Umemura; Tokuyama Central Hospital: Hiroshi Ogawa, Takahiro Iwami; Tokushima University Hospital: Masataka Sata, Tetsuzo Wakatsuki; Tokushima Prefectural Central Hospital: Hiroyuki Fujinaga, Akihiro Saito; Zentsuji National Hospital: Hisanori Shinohara; KKR Takamatsu Hospital: Yuichiro Takagi; Ehime University Hospital: Hideki Okayama, Jun Aono; Ehime Prefectural Niihama Hospital: Shozo Sueda, Tomoki Sakaue; Shinsenkai Daiichi Hosptal: Hiroshi Fujita; Chikamori Hospital: Kazuya Kawai, Syuuichi Seki; Kokura Memorial Hospital: Masashi Iwabuchi, Shinichi Shirai; Fukuoka Kinen Hospital: Nobuaki Takahashi, Hideki Fujiwara; Saiseikai Futsukaichi Hospital: Shinichi Ando, Toshiaki Kadokami; Fukuoka Wajiro Hospital: Taro Saito, Keita Nakamura; Saga University Hospital: Yutaka Hikichi; Ureshino Medical Center: Shiro Hata; Kouseikai Hospital: Yoshihiro Iwasaki; Japanese Red Cross Kumamoto Hospital: Yasuhiro Ogata, Ryusuke Tsunoda; Saiseikai Kumamoto Hospital: Kenji Horiuchi, Shinji Tayama, Naoko Takahashi; Kumamoto Chuo Hospital: Katsuo Noda; Nankai Hospital: Ken-ichiro Ito; Saiseikai Hita Hospital: Hitoshi Otsubo, Toshifumi Shimada; Oita Cardiovascular Hospital: Tadafumi Akimitsu; Miyazaki Medical Assocation Hospital: Yoshisato Shibata, Mitsuhiro Shimomura; Kanoya Heart Center: Hidekazu Arai; Kagoshima Medical Center: Kazuhiko Nakamura, Hitoshi Nakajima; Nanpuh Hospital: Shinichiro Toyoshima; Tenyoukai Central Hospital: Yoshihiko Atsuchi, Hiroshi Yamaguchi; Northern Okinawa Medical Center: Hiroki Uehara; Hadano Red Cross Hospital: Reimin Sawada; Tsukazaki Hospital: Yoshihiko Fu, Hidetaka Iida; Kagoshima Medical Center: Masahiro Sonoda, Katsuro Kashima; Dokkyo Medical University Hospital: Shichiro Abe.

