
Abstract
Aims: Our aim was to evaluate the incidence and clinical outcomes of late-acquired incomplete stent apposition (LAISA) after implantation of first- and second-generation drug-eluting stents in patients with acute myocardial infarction (AMI).
Methods and results: Late-Acquired incomplete stent aPPOsition after everolimus-eluting stent versus sirolimus-eluting Stent ImplanTatION in pAtients with non ST-segment elevation Myocardial Infarction and ST-segment elevation myocardial infarction (APPOSITION-AMI) was a prospective, randomised study comparing LAISA after everolimus-eluting stent (EES) and sirolimus-eluting stent (SES) implantation in AMI patients. Intravascular ultrasound examination was serially performed post-procedurally and at eight-month follow-up in 195 AMI patients (205 native coronary lesions: 100 EES; 105 SES). LAISA was observed in 6.0% and 16.2% of EES- vs. SES-treated lesions (p=0.021), respectively. In 64.7% of SES-treated lesions, LAISA was caused by positive remodelling, whereas thrombus dissolution or plaque reduction was observed in 66.7% of EES-treated lesions. Among patients with LAISA, MACE developed in one (4.5%) in the SES group with no ST in either group up to one year.
Conclusions: The incidence of LAISA was lower in AMI patients treated with EES as compared to SES, mainly secondary to positive remodelling in SES- but not EES-treated lesions. Patients with LAISA in both groups showed a very low MACE incidence at one-year follow-up.
Introduction
Although drug-eluting stents (DES) are highly efficacious at neointimal hyperplasia inhibition1, stent thrombosis (ST) remains a major concern2. Among the multiple patient-, lesion-, and procedure-related risk factors which may contribute to ST development2, late-acquired incomplete stent apposition (LAISA) has been associated with late or very late ST in the DES era3. In recent studies of de novo coronary lesions, there appears to be a lower frequency of LAISA in second- compared to first-generation DES1. Acute myocardial infarction (AMI) also increases the risk of ST2 and may be secondary to stent struts coming directly into contact with ruptured plaque and its highly thrombogenic necrotic core, leading to platelet recruitment4 or to DES-induced delayed vascular healing and local inflammation4,5.
From a clinical standpoint, clinical outcomes of LAISA remain a matter of debate3,6, and ST incidence does not appear unusually high in AMI patients after DES implantation7. Furthermore, limited data are available on LAISA development after second- versus first-generation DES in AMI patients. The present study aimed to evaluate the incidence, mechanism and one-year clinical outcomes of LAISA detected at eight-month follow-up after implantation in AMI patients of everolimus-eluting stents (EES) versus sirolimus-eluting stents (SES).
Methods
STUDY POPULATION
Late-Acquired incomplete stent aPPOsition after everolimus-eluting stent versus sirolimus-eluting Stent ImplanTatION in pAtients with non ST-segment Myocardial Infarction and ST-segment myocardial infarction (APPOSITION-AMI) was a multicentre, prospective, open-label, randomised controlled study comparing LAISA after implantation of an EES (XIENCE V; Abbott Vascular, Santa Clara, CA, USA, also distributed as the PROMUS stent; Boston Scientific, Marlborough, MA, USA) versus an SES (CYPHER®; Cordis Corp., Johnson & Johnson, Miami Lakes, FL, USA) in patients with AMI. All patients were randomly assigned (1:1) to receive either an EES or an SES. The study protocol was approved by the appropriate institutional review board/ethical committee of the respective clinical site, and written informed consent was obtained from all patients before an invasive procedure.
The present study included patients aged 18 years or older with AMI (ST-segment elevation myocardial infarction [STEMI] or non-ST-segment elevation myocardial infarction [NSTEMI]) undergoing percutaneous coronary intervention within 72 hours after hospitalisation. Patients were excluded if they had: 1) hypersensitivity or contraindication to either heparin or aspirin, 2) cardiogenic shock, 3) left ventricular ejection fraction <30%, 4) left main disease, 5) in-stent restenosis at target vessel, or 6) renal dysfunction (creatinine >2.0 mg/dL).
All patients were scheduled for coronary angiography with IVUS examination at eight months after the index procedure, and an additional one-year clinical follow-up was performed to evaluate the presence or absence of LAISA by IVUS.
INTERVENTION PROCEDURES
All patients received an intravenous bolus dose of heparin 10,000 U before the procedure and pre-treatment with antiplatelet drugs. All patients were given loading doses of 200-300 mg of aspirin and 300-600 mg of clopidogrel before coronary intervention and a daily dose of 100 mg aspirin and 75 mg clopidogrel after the procedure. Triple antiplatelet therapy was defined as 100 mg aspirin and 75 mg clopidogrel daily and 100 mg cilostazol twice a day. A reduced left ventricular ejection fraction was defined as LVEF <50% by Simpson’s method.
ANGIOGRAPHIC ANALYSIS
Quantitative coronary angiographic analysis was performed before and after stenting and at eight-month follow-up with an automated edge-detection system (Quantcor QCA, version 4.0; Pie Medical Imaging, Maastricht, The Netherlands). Late lumen loss was calculated as the post-intervention minimum lumen diameter (MLD) minus the MLD at follow-up. Angiographic binary restenosis was defined as diameter stenosis of ≥50% within the treated site at follow-up.
IVUS IMAGING AND ANALYSIS
IVUS examination was conducted post procedure and at eight-month follow-up after the index procedure using the same commercially available ultrasound systems with a catheter (iLab, Galaxy and 40-MHz Atlantis SR Pro catheter; Boston Scientific Corp., Fremont, CA, USA, or In-Vision Gold and 20-MHz Eagle Eye catheter; Volcano Therapeutics, Rancho Cordova, CA, USA). IVUS images were archived onto CD-ROM and sent to an independent IVUS core laboratory (Keimyung University Dongsan Medical Center, Daegu, South Korea) for off-line analysis by independent individuals blinded to treatment strategy.
Stent underexpansion was defined as minimal stent area <5 mm2 or a cross-sectional area (CSA) ≤90% of the distal reference lumen for small vessels8. Adjunctive balloon inflations were performed at the physician’s discretion if the IVUS criteria of stent underexpansion were met. IVUS images were analysed with commercially available quantitative volumetric analysis software (echoPlaque; INDEC Medical Systems, Inc., Mountain View, CA, USA). Incomplete stent apposition (ISA) was defined as lack of contact between stent struts and the underlying vessel wall, with evidence of blood speckle behind the struts in a segment not associated with any side branches9. Acute ISA was defined as ISA detection immediately after stent deployment. According to presentation timing, i.e., post procedure and/or at eight-month follow-up IVUS examination, ISA was classified into three categories: 1) resolved ISA, present at post procedure but not at follow-up; 2) persistent ISA, present at both post procedure and follow-up; and 3) LAISA, absent post procedure but present at follow-up9.
Lumen, stent, and vessel contours were traced with 1.0 mm axial intervals at the stented and reference vessel segments (defined as a 5 mm segment proximal or distal to the stent) and ISA section. In stented segments, the neointimal contour was additionally traced in the follow-up IVUS. Plaque area was calculated as vessel area minus stent area, and neointimal area as stent area minus lumen area. For volumetric analysis, volumes (lumen, stent, plaque, vessel, intimal and ISA) were calculated by means of Simpson’s rule. To adjust for stent length, each volume was divided by stent length (expressed as mm2). Percent neointimal hyperplasia (NIH) was calculated as the ratio between the NIH area and stent area ×100. Within LAISA segments, the following parameters were evaluated: 1) ISA volume; 2) ISA mean area; 3) ISA length; 4) ISA maximal depth; 5) the arc of ISA; and 6) presence of calcium at the ISA site. Positive remodelling was defined as an increase in EEM CSA from baseline to follow-up IVUS examination, whereas a decrease in plaque plus media (P+M) CSA from baseline to follow-up IVUS examination was considered as thrombus dissolution or plaque reduction. Tissue protrusion was defined as protrusion of tissue between stent struts towards the lumen on IVUS imaging. When ISA was detected at follow-up IVUS, a post-procedural IVUS review was carried out to identify the corresponding image slice to determine whether the ISA at follow-up IVUS was persistent or late-acquired. Axial landmarks (e.g., side branches, calcium deposits, stent edges) in both reference and stented segments were used to identify a matched site from baseline and follow-up IVUS studies.
To evaluate the reproducibility of IVUS measurements, 20 pullback images were randomly selected. For mean vessel area and mean lumen area, respectively, overall inter-observer agreement rates were 0.997 and 0.995, and intra-observer agreement rates were 0.999 for both. The mean±SD of the differences between measurements for vessel area and lumen area were 0.27±0.41 and 0.05±0.26 in inter-observer variability (Appendix Figure 1) and 0.12±0.26 and 0.10±0.12 in intra-observer variability, respectively.
STUDY ENDPOINTS
The primary endpoint was incidence of LAISA at eight-month follow-up after DES implantation. The secondary endpoints were cardiac death, non-procedure-related myocardial infarction (MI), target lesion revascularisation (TLR), angiographic late lumen loss; a cumulative incidence of major adverse cardiac events (MACE) including cardiac death, MI, or TLR and ST (defined as definite evidence of a thrombotic event according to Academic Research Consortium criteria)10.
Statistical analysis
Analyses were performed using SPSS statistical software, Version 20.0 (IBM Corp., Armonk, NY, USA). Quantitative data are presented as mean±SD, and qualitative data as frequencies. On the basis of previous studies, we assumed LAISA incidence to be 7% in the EES arm and 20% in the SES arm11-13. The sample size calculation was based on a two-sided type I error rate α of 0.05, a 1:1 (everolimus:sirolimus) ratio, and a statistical power of at least 80% to detect about 20% reduction, resulting in a total enrolment of 262 patients (131 patients in each group) in the present study. Comparisons for categorical variables were analysed using chi-square or Fisher’s exact tests, and those for continuous variables using the Student’s t-test or Mann-Whitney U test.
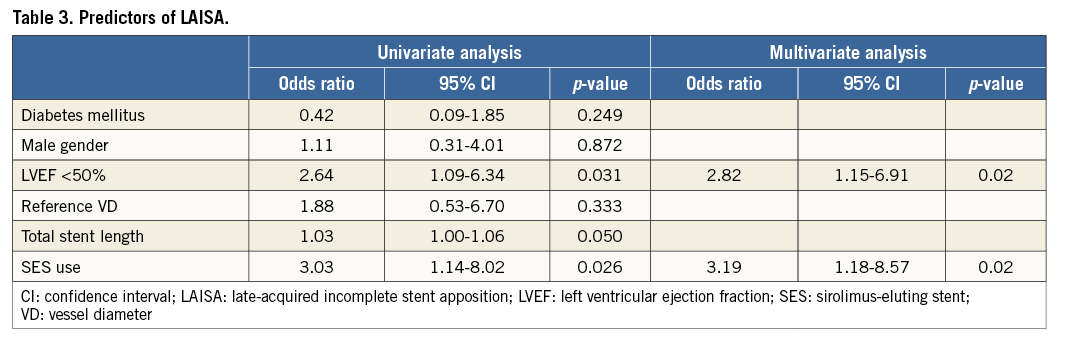
To determine independent predictors of LAISA, clinical, angiographic, and procedural variables with a value of <0.05 on univariate analysis were entered into multivariate logistic regression analysis. A p-value <0.05 was considered statistically significant.
Results
BASELINE CHARACTERISTICS
Patient, angiographic, and procedural characteristics are listed in Table 1 and Appendix Table 1. Angiographic follow-up and repeated IVUS examination at eight months were performed in 214 of 262 patients (81.5%). Of these, 19 patients were excluded (five with protocol violations, 10 with poor imaging quality, and four with inappropriate pullback length). Thus, 195 patients (96 EES, 99 SES) with 205 de novo lesions (100 EES, 105 SES) were analysed in both post-procedure and follow-up IVUS and completed a clinical follow-up.
LATE-ACQUIRED INCOMPLETE STENT APPOSITION
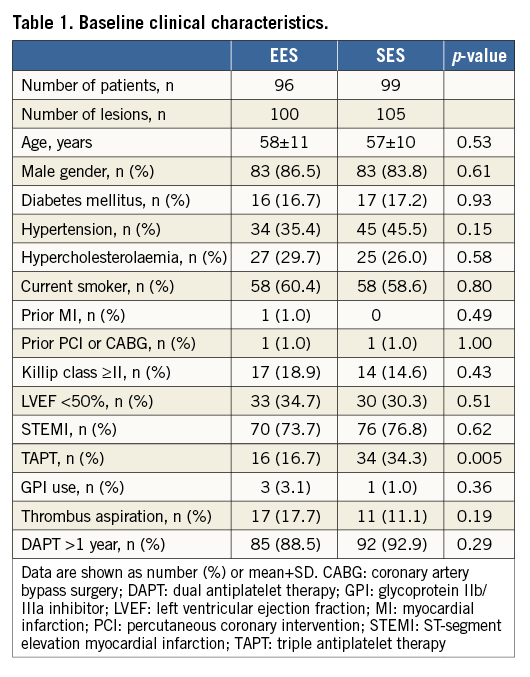
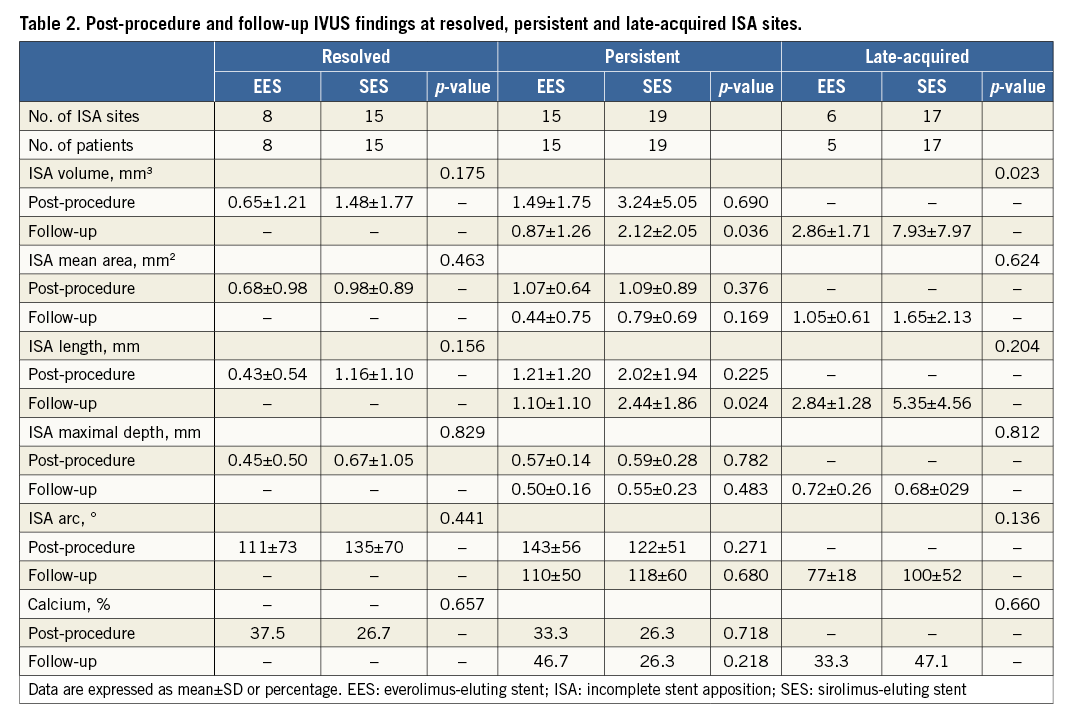
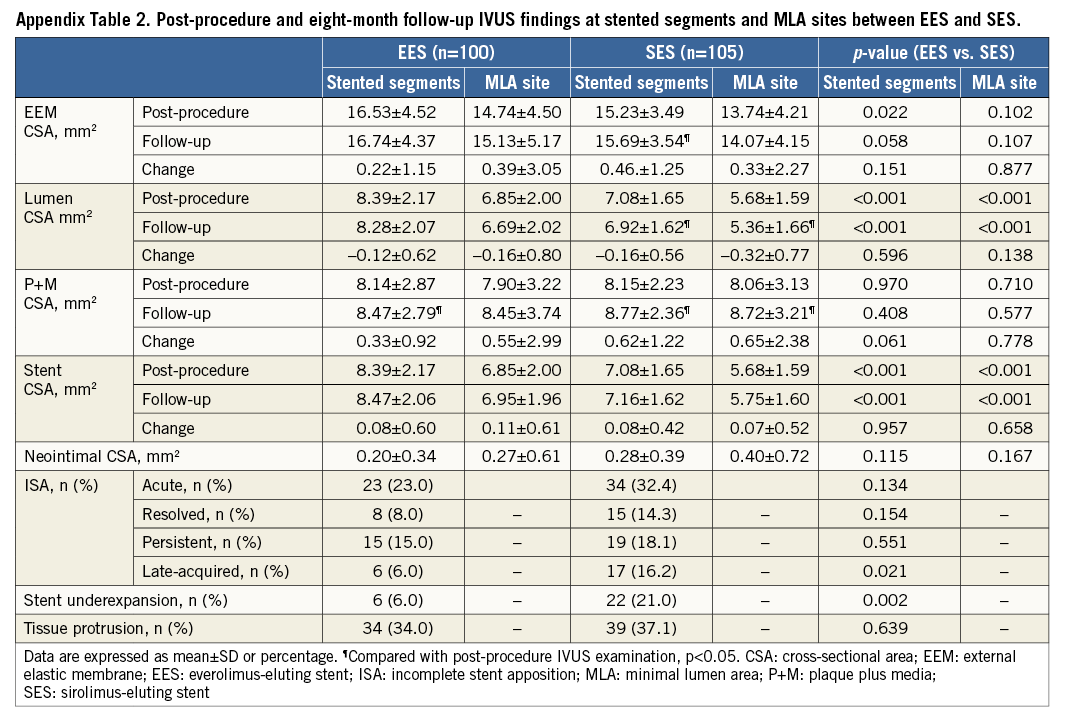
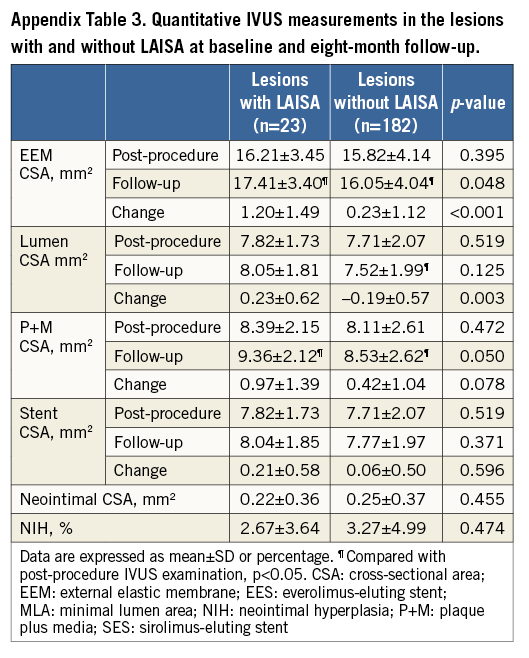
LAISA was identified at eight-month follow-up in six of 100 (five patients) EES-treated lesions (6.0%) and 17 of 105 (17 patients) SES-treated lesions (16.2%) (Figure 1, Figure 2). Post-procedure and eight-month follow-up IVUS findings at stented segments and MLA sites for EES and SES are shown in Appendix Table 2 and the characteristics of the 23 sites with LAISA in Table 2. Of these, two lesions in the EES group had multiple LAISA sites. Seventy-eight percent of LAISA sites were located within the stent bodies and 22% along the edges. There was no statistical difference between DES type and location of LAISA sites. LAISA sites had a significantly larger volume in the SES group compared with the EES group (7.9±8.0 mm3 vs. 2.9±1.7 mm3, p=0.023). During the follow-up period, at LAISA sites, positive remodelling (ΔEEM CSA increase) was apparent in two of six EES-treated lesions (33.3%) and thrombus dissolution or plaque reduction (ΔP+M CSA decrease) in four lesions (66.7%). In contrast, 11 of 17 SES-treated lesions (64.7%) showed positive remodelling, and six lesions (35.3%) had thrombus dissolution or plaque reduction during follow-up (Figure 1, Figure 2). After implantation of both types of DES, Δlumen CSA correlated with ΔEEM CSA (R=0.87, p=0.024 for EES, and R=0.82, p<0.0001 for SES) at the LAISA sites. However, when focused on LAISA sites with thrombus dissolution or plaque reduction, Δlumen CSA did not correlate with ΔEEM CSA in either group. Quantitative IVUS measurements in the lesions with and without LAISA at baseline and eight-month follow-up are shown in Appendix Table 3.
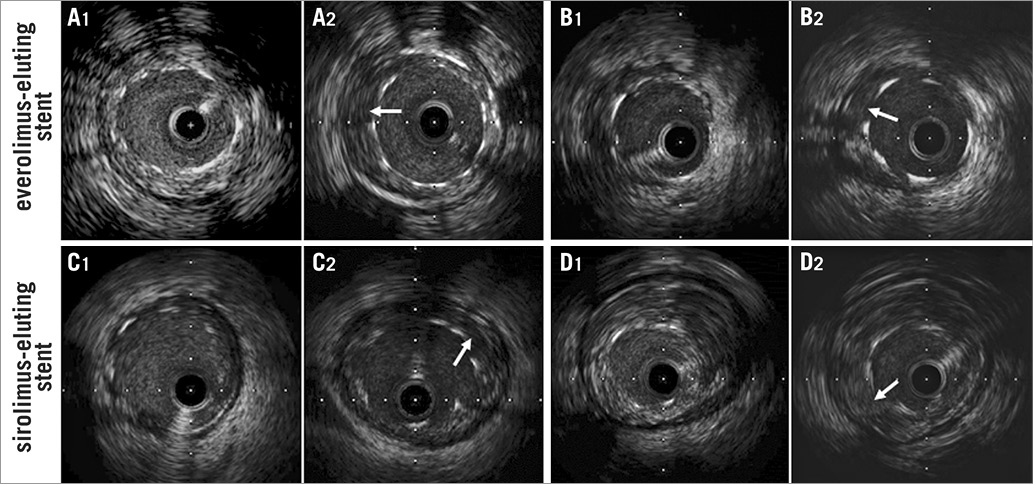
Figure 1. Illustrative IVUS images of late-acquired incomplete stent apposition (LAISA) after everolimus-eluting stent (EES) and sirolimus-eluting stent (SES) implantation. At the time of stent implantation, all stent struts were well apposed to the vessel wall (A1, B1, C1 and D1). However, incomplete stent apposition (arrow) was observed mainly due to positive remodelling (A2 and C2) and thrombus dissolution or plaque reduction (B2 and D2) at eight-month follow-up. Quantitative IVUS data are shown as follows: (B1 and B2) LAISA in EES-treated cases secondary to thrombus dissolution or plaque reduction (Stent CSA: 6.98 mm2, ISA CSA: 1.61 mm2, Δ EEM CSA: 0.97 mm2, Δ P+M CSA: –0.66 mm2, Δ lumen CSA: 0.01 mm2), (D1 and D2) LAISA in SES-treated cases secondary to thrombus dissolution or plaque reduction (Stent CSA: 6.68 mm2, ISA CSA: 1.34 mm2, Δ EEM CSA: 0.5 mm2, Δ P+M CSA: –1.28 mm2, Δ lumen CSA: 0.41 mm2).
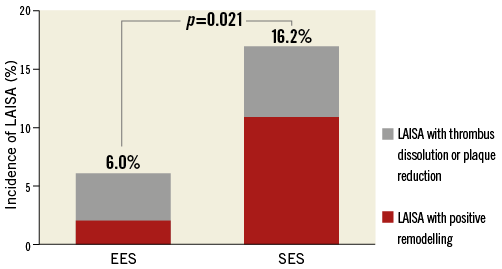
Figure 2. Incidence of late-acquired incomplete stent apposition (LAISA) after everolimus-eluting stent (EES) and sirolimus-eluting stent (SES) implantation in 195 AMI patients with 205 native coronary lesions (100 EES and 105 SES). In patients with LAISA with EES, four of six lesions were caused by thrombus dissolution or plaque reduction, whereas 11 of 17 lesions developed from positive remodelling in patients receiving SES.
Multivariate adjusted analysis revealed that SES use (OR 3.19, 95% CI: 1.18 to 8.57; p=0.02) and LVEF <50% (OR 2.82, 95% CI: 1.15 to 6.91; p=0.02) were independent predictors of LAISA after DES implantation in patients with AMI (Table 3).
ACUTE, RESOLVED, AND PERSISTENT INCOMPLETE STENT APPOSITION
For details of acute, resolved and persistent incomplete stent apposition, please refer to the Appendix.
ONE-YEAR CLINICAL OUTCOMES
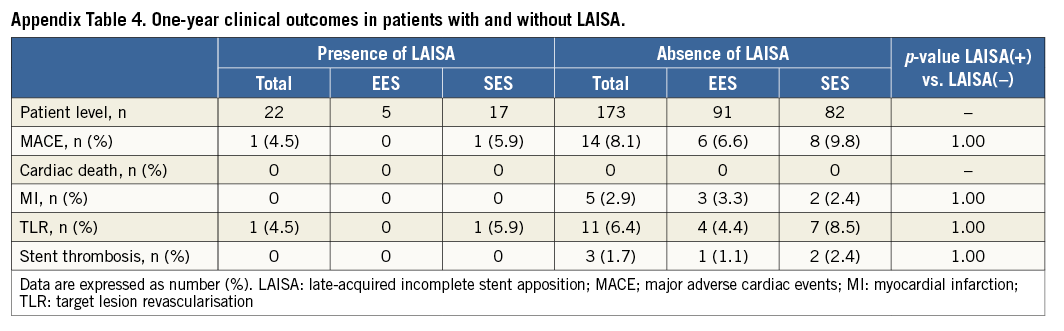
In 22 patients with LAISA (five EES, 17 SES), there was neither cardiac death nor non-procedure-related MI, and one patient treated with SES had TLR at one-year follow-up. No stent thrombosis was observed in either group. When comparing patients with and without LAISA (91 EES, 82 SES), cumulative MACE and stent thrombosis rates were not significantly different (Appendix Table 4).
Discussion
The present study investigated the development of LAISA and its clinical outcomes after second- versus first-generation DES implantation in AMI patients. The main findings of the present study were: 1) LAISA was observed less frequently in EES- than in SES-treated lesions; 2) positive remodelling was likely to occur in LAISA with SES relative to EES; 3) LAISA after EES and SES implantation appears not to be associated with cumulative MACE during one-year follow-up; and 4) SES use and reduced left ventricular ejection fraction were independent predictors of LAISA.
INCIDENCE OF LAISA
The present study demonstrated that LAISA detected by IVUS was observed in 16.2% of SES-treated lesions at eight-month follow-up in patients with STEMI and NSTEMI. In contrast, the incidence of LAISA has been reported to be 25% to 30% after SES or paclitaxel-eluting stent (PES) implantation in patients with STEMI11. The different study population characteristics might have influenced the lower incidence of LAISA in the present study.
On the other hand, limited data are available on LAISA after EES implantation. IVUS results from the SPIRIT III trial reported LAISA in 1.7% of 115 EES-treated lesions and in 4.3% of 46 PES-treated lesions at eight-month follow-up1. In the present study, 6.0% of EES-treated lesions developed LAISA during eight-month follow-up in AMI patients. Intriguingly, everolimus induces a selective removal of macrophages by autophagy that may have contributed to peri-stent vascular stabilisation in a rabbit model14. Recent serial optical coherence tomography (OCT) studies reported favourable vascular response after stent implantation within one year of follow-up, which supports our result15. Follow-up OCT findings have shown lower incidences of uncovered struts, intracoronary thrombi and malapposed struts in EES compared to those in SES15. Taken together, LAISA is less likely to occur after second-generation EES implantation compared to first-generation SES implantation, as supported by the achievement of statistical significance in LAISA occurrence difference between EES and SES (16.2% vs. 6.0%, p=0.021) in the present study. However, procedural factors such as one third of adjunctive balloon inflations at the physician’s discretion may explain the lower incidence of LAISA with both stents as compared to previous studies9,11,16.
MECHANISMS AND PREDICTORS OF LAISA
Several investigators have suggested that the main mechanism of LAISA is positive remodelling with or without an increase in plaque area regardless of the patient’s clinical presentation, especially following DES implantation9,11,12,16. The HORIZON-AMI IVUS substudy reported that more than 70% of 62 LAISA in PES-treated patients were caused by positive remodelling, and 84% of 45 LAISA in SES-treated patients had the same vascular behaviour in the MISSION IVUS substudy11,16. These findings suggested that LAISA in patients with STEMI were also caused by positive remodelling. In the present study, positive remodelling was observed in 11 of 17 lesions (64.7%) in the SES-treated arm, which is in line with previous studies11,16. Compared to SES, the underlying mechanism of LAISA after EES implantation was not as readily apparent, probably due to low event frequency1. In the SPIRT III trial, two lesions developed LAISA at eight-month follow-up without vessel enlargement1. Although dissolution of the thrombus or plaque reduction was more frequently observed (four of six lesions) in the present study, the low LAISA incidence in EES-treated lesions precludes drawing any conclusions.
In the present study, SES use and reduced LVEF were independent predictors of LAISA. Numerous studies have reported that independent predictors of LAISA in patients with coronary artery disease were stenting in unstable angina or AMI, lesion or total stent length, absence of diabetes, CTO lesions, and DES use after bare metal stent (BMS) or DES implantation11,12,16. IVUS substudies from MISSION and HORIZON-AMI demonstrated that the use of SES or PES and plaque/thrombus protrusion were associated with LAISA risk in STEMI patients11,16. Thick stent struts and/or less biocompatible polymer in first-generation DES, namely SES or PES, may provide a potential source for hypersensitivity reactions in coronary lesions, resulting in a higher chance of positive remodelling in the stented segments compared to second-generation DES or BMS. On the other hand, culprit lesions in AMI or unstable angina may be accompanied by thrombus. During the follow-up period, thrombus-containing lesions are at risk for thrombus dissolution9,11,16 and LV dysfunction in the presence of high thrombus burden17, which would contribute to LAISA development in AMI patients.
CLINICAL OUTCOMES OF LAISA
The clinical relevance of LAISA has been a matter of debate. During additional follow-up up to one year, there was no ST in patients with LAISA in either the EES or the SES group in the present study, which may be explained by a higher frequency (almost 90%) of dual antiplatelet therapy beyond one year in both groups. In addition, TLR was observed in only one patient with LAISA after SES implantation, translating into no difference between the EES and SES groups in terms of MACE. Many investigators have reported no negative impact of LAISA on mid- to long-term clinical outcomes, which is in agreement with our observations6,12. A pooled analysis from the RAVEL, E-SIRIUS and SIRIUS studies showed similar MACE rates in SES-treated patients with and without ISA (11.1% of 45 patients vs. 16.3% of 135 patients, p=0.48) during four years of follow-up6. Similar observations were also made in patients with LAISA in the MISSION and HORIZON-AMI substudies during one year of follow-up11,16. In contrast, a study by Cook et al demonstrated a negative impact of LAISA following DES implantation on long-term clinical outcome3. In addition, a meta-analysis of 17 trials suggested that LAISA may be associated with an increased risk of ST18. However, based on the currently available data, the clinical relevance of LAISA is far from conclusive. Further large-scale investigation with longer-term follow-up is needed.
Limitations
The present study has several potential limitations. First, the low LAISA incidence, despite the prospective study design, may not provide enough power to detect differences in the underlying mechanisms of LAISA between the two stents. In particular, the low LAISA incidence in EES-treated lesions precludes drawing any conclusions. Second, the same antiplatelet regimen with treatment duration was not mandatory in all patients, which may have contributed to the different incidence of LAISA between the EES and SES groups. Third, the small sample size of the population and the short follow-up period after detection of LAISA may not provide firm conclusions regarding the clinical impact of the LAISA. Fourth, the patient drop-out rate in this study was slightly higher than we expected, translating into the possibility of the study being underpowered to detect differences in the incidence and underlying mechanisms of LAISA between the two stents. Finally, there was a trend towards a higher incidence of acute ISA in SES- as compared to EES-treated patients. Any association between the difference in lesion treatment observed at baseline and the finding of a higher frequency of LAISA cannot be excluded.
Conclusion
In patients with AMI, the occurrence of LAISA was less frequent after EES implantation as compared to SES. In addition, positive remodelling was more frequently observed in LAISA with SES, suggesting that improved biocompatibility, most likely to be polymer-related, with EES might reduce the development of LAISA compared to SES. In this patient subset, LAISA after EES or SES implantation was not associated with clinical events during one-year follow-up.
| Impact on daily practice Late-acquired incomplete stent apposition has been reported to be a predictor for late or very late stent thrombosis and is mainly caused by positive remodelling following drug-eluting stent implantation. In patients with AMI, the occurrence of LAISA was less frequent in patients treated with EES as compared to SES, and positive remodelling was more common in LAISA with SES. These findings suggested that better biocompatibility with EES compared to SES may reduce the development of LAISA in the setting of AMI. |
Acknowledgements
We are grateful to Chang-Wook Nam, Yun-Kyeong Cho, Hyuck-Jun Yoon, Hyoung-Seob Park, Hyungseop Kim, Seongwook Han, Yoon-Nyun Kim, and Kwon-Bae Kim (Keimyung University Dongsan Hospital) for their critical support and advice.
Conflict of interest statement
The authors have no conflicts of interest to declare.
SUPPLEMENTARY DATA
Appendix. Acute, resolved, and persistent incomplete stent apposition
At the index procedure, acute ISA was observed in 24.0% (24 out of 100) of lesions in the EES group and in 34.3% (36 out of 105) of lesions in the SES group (p=0.11). One case of multiple sites acute ISA was noted in each stent group. Among 24 acute ISA lesions in the EES group, eight lesions (33.3%) resolved and 16 (66.7%) persisted at eight-month follow-up IVUS examination. In the SES group, resolved ISA was detected in 15 out of 36 lesions (41.7%) and persistent ISA in 21 out of 36 lesions (58.3%). The incidence of resolved and persistent ISA was not different between the EES and SES groups (p=0.52). The characteristics of 34 sites with persistent ISA (15 in EES, 19 in SES; three were excluded because of location in the left main artery) are shown in Appendix Table 4. Compared to the EES group, the SES group had significantly larger volume (2.1±2.0 mm3 vs. 0.9±1.3 mm3, p=0.04) and greater length (2.4±1.9 mm vs. 1.1±1.1 mm, p=0.02), probably secondary to discrepancy in commercially available stent sizes or stent designs.
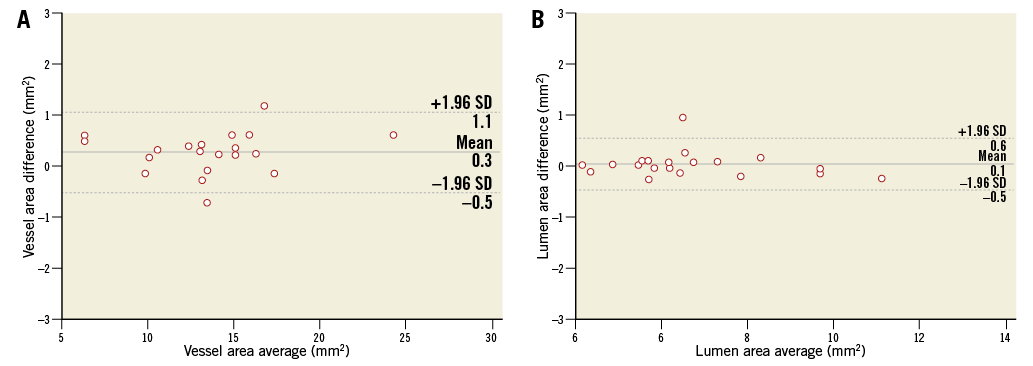
Appendix Figure 1. Bland-Altman analysis. Inter-observer agreement for the vessel (A) and lumen area (B). Bland-Altman plots showed no significant difference between two observers with good agreement.

