Abstract
Late acquired incomplete stent apposition (LAISA) is an intravascular ultrasound (IVUS) finding that has been associated with late and particularly very late drug-eluting stent (DES) thrombosis. The underlying mechanism appears to be regional positive remodelling due to inflammation necrosis related with DES components. Incidence is higher for DES (10-12%) compared to bare-metal stents (4%) in native vessels, with no data in saphenous vein grafts. Large LAISA has been found in 60-77% of cases with DES late thrombosis studied with IVUS. However, clinical follow-up studies of cases with routinely detected LAISA either do not show a relationship to thrombosis or only reflect a trend, although when taken as a whole, that trend is significant. There is no evidence that correction of LAISA should be a target for secondary or repeated revascularisation. Benefits and risks of LAISA correction are unknown.
Introduction
The basic primary mechanism underlying late stent thrombosis, as shown by autopsy studies in humans with both bare-metal stents (BMS) and drug-eluting stents (DES), is the lack of endothelialisation of stent struts associated with fibrin deposits1,2. This delayed healing phenomenon is significantly more frequent with DES than with BMS, which appears logical when the mechanism of action of anti-proliferative drugs are taken into account. The imaging technique most frequently used in clinical practice to evaluate cases with stent thrombosis is intravascular ultrasound. In cases of early DES thrombosis, and in a similar way to studies with BMS, the main factors identified were stent under-expansion, stent length, dissection and/or residual disease at the stent edges3,4. The following have also been described to a lesser extent in association with the aforementioned factors: branch jailing, stent overlapping and incomplete stent apposition.
In studies focusing on cases with DES, late or very late thrombosis, stent length, overlapping and under-expansion have also been found to be predictors, although not as predominantly as in early thromboses. However, a new factor has come to light that seems to be specific to later thromboses and this is late-acquired incomplete stent apposition.
Incomplete stent apposition
Definition and types of incomplete apposition
Incomplete apposition is separation of at least one strut from the intimal surface of the arterial wall, not overlapping a side branch, with evidence of blood flow behind the strut(s).
There are several types of incomplete apposition depending on when it is diagnosed:
Acute: observed during the immediate post-implantation examination.
Late: observed at follow-up.
Late incomplete apposition can also be categorised as:
Persistent late: initially observed after implantation and was not corrected at that time.
Late-acquired: not observed at baseline but developed at a later date.
Acute
Acute incomplete apposition (acute ISA) has not been associated with sub-acute thrombosis as an isolated finding but in association with others such as sub-expansion. Acute ISA is especially observed at stent edges and tends to be discreet. It is associated with progressive-size treated segments in which the stent is well apposed to the smaller reference segment (usually the distal), but not the larger reference segment (proximal), or to calcified plaque that inhibits complete stent apposition on one of its edges by acting as a fulcrum. It is detected when the result is evaluated using IVUS, and in this case tends to be corrected with over-dilation, at least when obvious. However, if no IVUS is conducted, it may go unnoticed.
Ultrasound follow-up studies have shown resolution of most of these cases and in persistent cases; such studies have not revealed an association between acute ISA and adverse events5-7. One thing that has been observed is less neointimal hyperplasia of the persistently non-apposed struts8. It is speculative to suppose that this persistent incomplete apposition is also associated with a lack of endothelialisation of the DES, something that only high-resolution techniques (OCT) can determine.
Late
This is incomplete apposition observed in a study with follow-up IVUS. If a baseline IVUS study is not available, it is impossible to differentiate acute-persistent from acquired incomplete apposition. Late-acquired incomplete stent apposition (LAISA) has been associated with late / very late DES thrombosis and will be considered in more detail below. This finding implies a continuum from one malapposed strut to coronary aneurysm formation. Figure 1 shows images of cases with late incomplete stent apposition.
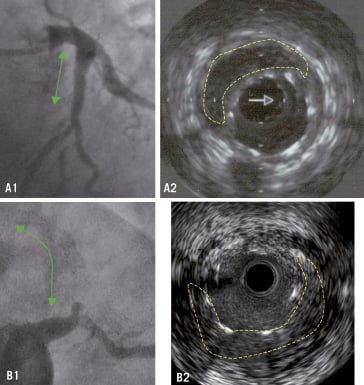
Figure 1. Cases of late incomplete stent apposition. As an incidental finding, A1: DES in proximal left anterior descending artery implanted 2 years ago. A2: IVUS showing no complete apposition. As a finding in thrombosis, B1: Very late thrombosis (3 years) in two long-overlapped DES in proximal mid-left anterior descending artery. B: IVUS showing large incomplete stent apposition. Traced in dashed lines the areas of no apposition. (Last case courtesy of Dr. F. Bosa from HUC, Tenerife, Spain).
Late-acquired incomplete stent apposition (LAISA)
Incidence has been determined in randomised or observational studies in which one IVUS study has been conducted during the initial procedure and another follow-up IVUS has been conducted after 6-9 months.
Limitations of IVUS in detecting LAISA
Sensitivity is high, but this may be slightly reduced, especially in mild cases, as a result of the area of malapposition being concealed due to reverberations caused by the highly echo-reflective metal struts. Second, it is likely, especially in cases with obvious stent thrombosis, or even without such thrombosis, that a certain amount of thrombotic tissue may fill the space of the incomplete apposition, which would then remain unnoticed or be underestimated. Given its excellent resolution and reduced reverberation around metal struts, the optical coherence tomography technique may increase sensitivity to detect this phenomenon.
Incidence with BMS
LAISA has already been described with BMS. In randomised trials, the incidence of LAISA after 6-9 months has been from 0 to 6%6,7,9-16. In observational studies, the incidence at six months was 4.4% in 206 patients and 5.4% in 881 patients17,18. In the first study, 88% were located at the stent margins while in the second, 67% affected the stent body. The incomplete apposition area was 3±2.1 mm2 in the BMS arm of TAXUS II6 and in both observational studies the area was 3.1±2.4 mm2 and 3.8±2.1 mm2.
Late incomplete apposition observed after brachytherapy deserves separate consideration. Variable incidences were reported, ranging from 9 to 22% with phosphorus-32 radioactive stent and phosphorus-23 radiation respectively19,20. At that time, this was one of the mechanisms potentially involved in the incidence of late thrombosis observed with these therapeutics.
Incidence with DES
Sirolimus-eluting stent (SES)
In the RAVEL study, no baseline IVUS was carried out, so it was not possible to determine to what extent the late apposition was acquired or persistent. However, after 6-12 months apposition was observed more with SES (21% vs. 4%)21. In the SIRIUS study, a baseline IVUS was carried out and LAISA was observed in 8.7% of patients with SES and 0% with BMS7. LAISA was located in 78% of patients in the stent body. In the DIABETES study, the results showed 15% with SES vs. 0% with BMS11. In the MISSION study, the incidence in ST-segment elevation myocardial infarction was 25% vs 5%10.
In observational studies, the incidence was 13% in a series of 538 stents and 4% in a series of 175 patients22,23. In the first study, apposition was central in 90% of cases and in the last study, apposition was located in the stent body in 84% of patients.
The incomplete apposition area in the RAVEL study was 2.5±1.8 mm2. This remained stable at an additional follow-up 12 months after the first (2.4±1.7 mm2)24. In the broadest observational study conducted by Hong et al, the incomplete apposition area was 3±1.9 mm2. 22
Paclitaxel-eluting stent (PES)
In the TAXUS II study, late apposition was observed in 8% of slow-release PES, 9.5% of moderate-release PES and 5.4% of patients with BMS6. At a 2-year follow-up on a subgroup of cases, a significant resolution of LAISA was observed with the incidence being reduced to 2.4% in the subgroup of 41 cases with moderate-release PES (from 9.8% at six months) and 0% in the 43 cases of slow-release PES (from 9.3% at six months)25. In the combined TAXUS IV, V and VI trials, the incidence of LAISA after nine months was 8.4% with PES (mainly the result of patients with moderate-release PES in TAXUS VI) and 3.5% with BMS13. In the SELECTION study, the incidence in primary angioplasty was 5% and 2.7% with BMS12. In the SPIRIT III study, the PES arm showed an incidence of 2%26. In the observational studies, late apposition was observed in 8.4% of 167 stents and in 15% of just 20 cases22,23. The incomplete apposition area in the TAXUS II study was 3.6±1.2 mm2 for slow-release PES and 2.1±1.4 mm2 for moderate-release PES with maximum areas of 5.1±1.8 and 3.4±2.6 mm2, respectively. In the Hong et al study, this mean area was 3.2±1.9 mm2. 22
Second-generation DES
In the ENDEAVOR II study, no case of LAISA was detected with zotarolimus-eluting stents14. In the ENDEAVOR RESOLUTE observational study, LAISA was observed in 6.8% of cases27. In the FUTURE I and II studies, the incidence was zero with everolimus-eluting stents and in the SPIRIT III study, it was just 1.1%15,26.
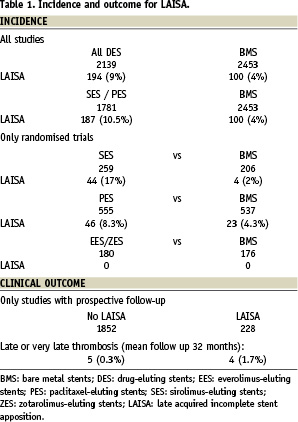
To summarise, the incidence with BMS is from 0 to 6% in trials and from 4 to 5% in registries. With sirolimus-eluting stents it is from 9 to 25% in trials and from 4 to 13% in registries. With paclitaxel-eluting stents, it is from 2 to 8% in trials and around 9% in real-world practice. With second-generation DES, considering that there are fewer studies available, the incidence with zotarolimus-eluting stents is from 0 to 7% and with everolimus-eluting stents is from 0 to 1%. Recently, two meta-analysis have been published concluding both with a higher incidence of LAISA with DES28,29. In the largest, analysing 17 studies (11 randomised and six observational), the OR for DES was 2.49 with CI 95% 1.15-5.35 (p=0.02)29. This risk was higher if only the randomised studies were analysed (OR 4.36, CI 95% 1.74-10.94, p=0.002). See Table 1 based on data from all trials and observational studies analysed. With regards to the magnitude of LAISA, this seems similar for both BMS and DES with a mean malapposition area of 3-4 mm2.
Of note, there is no data regarding incidence of LAISA in DES implanted in non-native vessels such as saphenous vein grafts.
Mechanisms and predictors for LAISA
There are three possible mechanisms for LAISA30,31
1)Regional positive remodelling. An increase in vessel area (external elastic membrane area) out of proportion to the increase in plaque area.
2)Thrombus dissolution or plaque regression behind the stent, especially the first. This could occur in lesions with a high thrombotic burden, such as lesions in infarction.
3)Chronic stent recoil (practically impossible).
Mechanisms and predictors for LAISA with BMS
In studies with BMS, it was observed that the underlying mechanism has been an increase in vessel area out of proportion to a modest increase in plaque area32. This is therefore a case of regional positive remodelling. In one of the studies, directional atherectomy before stenting and direct stent implantation in the infarction were determined as independent predictors with an incidence of LAISA of 10.3% and 11.5%18. This was attributed to deep arterial aggression induced by the atherectomy that would facilitate positive remodelling. In fact, a smaller pre-procedural minimal luminal diameter and a higher balloon/artery ratio were also observed in cases with LAISA. In the infarction case, thrombus dissolution under the stent was considered.
Mechanisms and predictors for LAISA with DES
In accordance with the studies with BMS, the increase in EEM area out of proportion to the increase in plaque or neointimal area has also been observed to be an essential mechanism for LAISA with DES6,7,13. A second, less significant, mechanism would be the dissolution of thrombotic material that in some cases (primary angioplasty) can contribute to the phenomenon.
Specifically with regards to DES, the group of Renu Virmani has described inflammatory changes and hypersensitivity reactions in the intima and media in thrombosis case autopsies with extensive vasculitis, apoptosis, eosinophil and lymphocyte infiltration and necrosis2. Chronic inflammation causes immune cells to release proteases-collagenases that weaken the wall and lead to its expansion. The effects of the polymer and drug in the DES would be added to the effects of the metal stent itself and the parietal damage induced by the angioplasty, thereby explaining the increased incidence of LAISA with DES in comparison to BMS.
With regards to mechanisms for LAISA, it is possible that there are some differences depending on the type of DES. Therefore, in the Hong et al study22, LAISA in cases of sirolimus-eluting stents was explained by both an increase in EEM area and greater neointimal suppression, while in cases with PES, remodelling was more pronounced. In fact, as indicated in the incidence section, a different pattern of evolution has also been seen for cases of LAISA depending on the type of DES. In the TAXUS II study, a highly significant resolution of malapposition was observed due to regression of the peri-stent area, not due to intimal proliferation25. This leads us to consider deferred healing of the arterial wall. However, in the RAVEL study, cases with late incomplete apposition observed at the six or 12-month follow-up and evaluated again 12 months later did not show changes with the same values for incomplete apposition and peri-stent area24. We must take into account that there was no baseline IVUS in this study and there could be cases of acute and persistent apposition among the cases of late apposition. Even so, these cases, as we have already seen, would not explain the persistence of the incomplete apposition as they tend to regress.
The greater inhibitory effect of sirolimus-eluting stents on proliferation may explain these differences or differential aspects in the actions of both drugs on the arterial wall that induce a different chronology in the healing process. It is likely that a hypersensitivity reaction to the drug and/or polymer prevails with SES while cytotoxicity prevails with PES.
In the TAXUS II study, lesion length, unstable angina and absence of diabetes were found to be predictors. In the pooled TAXUS IV, V and VI analysis stent length and infarction. In the combined RAVEL, E-SIRIUS and SIRIUS study, predictors were CCS class III or IV angina and absence of diabetes. Finally, in the Hong et al study22, primary stenting in infarction and chronic total occlusions. In this latter study, the incidence of LAISA was 31.8% after primary stenting in infarction, 27.5% after stenting in chronic total occlusion and 25% if directional atherectomy before stenting had been carried out. A relationship was also observed in this study, as with BMS, between a smaller minimal luminal diameter at baseline and a higher incidence of LAISA.
With regards to the absence of diabetes, its association could be explained by suppressed neointimal proliferation and reduced plaque progression. With regards to unstable angina and infarction, LAISA is associated with stenting in the presence of thrombus and in chronic occlusion it is associated with potential subintimal guidewire penetration with stent implantation in a false lumen and the consequent adventitial damage.
Relationship between incomplete stent apposition and BMS thrombosis
In the 1990s, studies were published showing baseline ultrasound observations of cases that later revealed thrombosis (usually sub-acute). The most frequent and consistent finding was under-expansion but in some studies, incomplete apposition was detected. The POST study33, found incomplete apposition in 49% of cases, while the usual result in cases without thrombosis is 4-21%. However, another study found incomplete apposition in a similar proportion to a control group (9% vs. 3%)34. In these two studies, the condition was obviously always acute and persistent incomplete apposition.
A study by Alfonso et al35 included direct IVUS examination of thrombosed stents. Incomplete apposition was observed in four out of 12 cases (33%). In these cases, the lack of a baseline IVUS study and the sub-acute nature of most of these thromboses lead us to assume that the incomplete apposition detected was basically acute and persistent.
Relationship between LAISA and late DES thrombosis
In this respect, there are two types of studies, those that evaluate cases of late thrombosis using IVUS or during autopsy, and those that report the clinical evolution of cases showing LAISA at routine follow-up controls.
Studies evaluating cases with thrombosis
The study by Joner et al2 evaluated autopsy examinations of 23 patients who had died with a DES implanted more than one month before, of which 14 showed thrombosis. A lack of endothelialisation was systematically found in these cases with thrombosis and incomplete apposition was found in two patients (14%).
Among IVUS studies, the study by Cook et al36 evaluated ultrasound findings in 13 patients with very late DES thrombosis. The results were compared with the results of a control group of 144 DES patients who underwent routine IVUS examinations at eight months and who did not show adverse events within two years of follow-up. The authors found a longer stent length, more stents per lesion, more stent overlapping (39% vs. 8%), less stent expansion (68% vs. 81%) and finally a greater incidence of late incomplete apposition (77% vs. 12%) in the cases with thrombosis. With regards to expansion, this finding is not easily assessable given the possible affect of positive remodelling on the reference segments; in fact, the minimum intra-stent area was similar. With regards to the aspect that is of interest to us, late incomplete apposition, the lack of a baseline IVUS study prevents us from determining whether such apposition was acquired or acute and persistent. Nevertheless, its large size and the incidence of late ISA in controls (12%), which is very comparable to LAISA observed in clinical practice studies, lead us to assume that it was mainly acquired.
Its magnitude was also significantly different in the two groups; the maximum malapposition area was 8.3±7.5 mm2 in cases with thrombosis, which is much higher than the area of 4±3.8 mm2 seen in controls and the area from studies with routine IVUS follow-up (2.5–5 mm2). In fact, three of the cases were authentic aneurysms. A 45% of appositions were located in the body of the stent, 36% were located in the proximal segment and 18% in the distal segment. With regards to clinical characteristics, it was observed that 46% of the cases with thrombosis were DES implantations in ST-segment elevation AMI compared to 26% of the controls and no cases with thrombosis were diabetic compared to 18%. These differences, although not significant given the reduced sample size, are worthy of consideration.
The second study by Alfonso et al37 included the evaluation of 12 cases of thrombosis (five being late thrombosis). The main finding was under-expansion. Incomplete apposition was detected in six cases (50%), three sub-acute and three late. Therefore, three of the five cases of late thrombosis (60%) presented incomplete apposition. We must once again consider that the lack of a baseline IVUS study.
In comparison with these studies, there are others that have not found a higher incidence of malapposition in cases of DES thrombosis. However, these studies only deal with sub-acute thrombosis, Fujii et al3, incomplete apposition in 13% of thrombosis compared to 16% in controls, or are based on baseline IVUS after initial procedure, Okabe et al38 with five late thrombosis out of 13, acute malapposition 0% vs. 6% in controls.
To summarise, studies of cases with late and very late thromboses reflect the frequent presence of LAISA, with this being of a large size, in fact clearly larger than the detected in routine studies.
Clinical follow-up studies of cases with routinely detected LAISA
Cases with LAISA in the RAVEL, SIRIUS and E-SIRIUS studies (45 with sirolimus-eluting stents) did not reveal a higher incidence of major adverse events in four years (11.1% vs. 16.3%), although they did have a trend towards more infarctions (11.1% vs. 4.4%) and late thrombosis (2.2% vs. 0%)9. In the TAXUS II study, 20 patients with paclitaxel-eluting stents and LAISA did not suffer thrombosis during one year of follow-up6. The pooled TAXUS IV, V and VI study with 24 cases with PES and LAISA did not report any thrombosis after two years of follow-up either13.
The observational study by Hong et al reported very late thrombosis in 1.3% of the 82 cases with LAISA and in 0.4% of cases without LAISA over three years of follow-up39. With regards to cardiac death, the same differences were reported (1.3% vs. 0.4%) Overall, an insignificant trend was reported for more death and infarctions.
In the Siqueira et al study23, two (20%) of the 10 patients with LAISA after DES implantation suffered thrombosis after a mean follow-up of 29 months compared to 0% of cases without LAISA. In comparison to those cases without events, these cases represented the most extreme cases of incomplete apposition with multiple malapposed segments of broad magnitude (incomplete apposition volume / vessel volume of 13.2±8.7% vs. 5.2±8.7%).
In the previously mentioned meta-analysis29, the clinical events of the 228 cases with LAISA from these studies were analysed, both with DES and BMS, along with the clinical events of the 1,852 cases without LAISA (Table 1). During a mean follow-up of 32 months (12 to 48 months), there was late or very late thrombosis in four of the 228 cases (1.7%) and in five of the 1852 cases (0.3%). The analysis conducted by the authors showed that LAISA implies a higher risk of late thrombosis (OR= 6.5, CI 95% 1.3-34.9, p=0.02).
Finally, as we have already indicated in the definition of LAISA, coronary aneurysms represent the extreme form of DES-related LAISA. In a series of 1,200 patients treated with DES and with a mean follow-up of 10 months, aneurysms were observed in 15 patients (1.25%), three (20%) of which had thrombosis40.
In conclusion, these clinical follow-up studies of cases with LAISA either do not show a relationship to thrombosis or only reflect a trend, although when taken as a whole, that trend is significant. Challenging this link is the fact that PES which develop less LAISA in follow-up have been associated to a higher incidence of late thrombosis in some meta-analysis and registries41,42. Also, the use of DES in infarction, a predictor for LAISA, although being a predictor for late thrombosis in registries42,43 it has not been yet associated with more thrombosis in trials with 1-2 years follow-up44.
Mechanisms that explain the association between LAISA and thrombosis
The incomplete apposition area may serve as a niche for platelet and fibrin deposits. Positive remodelling would facilitate a more static flow between the stent and the arterial wall and this arterial wall may show inflammatory alterations and necrosis, which are the cause of the remodelling and make its surface more thrombogenic2,36. To this we could add poorer endothelialisation of the struts; in fact, incomplete apposition has been associated with less neointimal hyperplasia, both with BMS and DES17,18,22. It is also possible that malapposition is basically a marker of other, associated, primarily more thrombogenic mechanisms, such as the lack of endothelialisation, vasomotor dysfunction or chronic inflammation.
LAISA and secondary revascularisation: what to do in the case of LAISA, with or without associated thrombosis?
LAISA may be observed during a routine follow-up examination or during a procedure on a DES with thrombosis. In both cases, we would ask ourselves whether the malapposition should be corrected but there is no evidence whatsoever supporting this.
Incidental finding
In the case of routine examination, we have seen throughout this study that although LAISA is not infrequent (around 10%), thrombosis is (< 2% over almost three years) and such thrombosis occurs in its most extreme, almost or clearly aneurysmal forms. Therefore, most are incidental findings that will never have repercussions.
The larger LAISA seem to entail more risk but correcting them would entail over dilating the original stent to a diameter of 2 mm or more, without being able to correct the wide malappositions described in cases of thrombosis. This, besides being ineffective, may be harmful as the stents would rupture and become distorted, and the arterial wall, surely weaker and thrombogenic, portends more risk of rupture or dissection. The remodelling process could even start up again and, moreover, the degree of endothelialisation could hardly be changed.
In the event of a clear aneurysm, the risk of thrombosis seems to be very high (20%) and one alternative would be a PTFE stent-graft.
Findings in thrombosis
LAISA can be detected in 60-77% of late thromboses and we know that late thromboses show certain recurrence, 3.3% at one year in ESTROFA study43. In these cases, the necessary re-dilatation of the stent could make it more tempting to try to eliminate the eventually detected incomplete apposition but the difficulties and risks involved would be as indicated above and its effectiveness is unknown. Once again, in the event of an aneurysm, implantation of a stent-graft may be considered.
Although there is no evidence supporting mechanical treatment of LAISA, diagnosis of LAISA may have implications on medical treatment, specifically to maintain combined anti-platelet treatment for an indefinite time in cases with a non-high bleeding risk and to obviously avoid suspending treatment in the event of surgery.
Is a randomised study feasible for treatment of incidental LAISA?
The design of a randomised study on whether LAISA should be treated does not appear feasible given the following considerations:
LAISA is not rare, but it is also not very frequent; very late thrombosis is really infrequent, 0.3-0.6% up to the 4th year and the risk may decline after the 3rd 45; in 2-3 years < 2% of cases with LAISA develop thrombosis. Hence, a very broad study would be necessary with baseline and follow up IVUS examinations in all patients and a very long clinical follow-up period.
Active search for LAISA?
Patients with DES who are going to undergo an operation months or years after implantation could be candidates for an evaluation with IVUS or a non-invasive CT-scan examination to detect LAISA and, if applicable, recommend not stopping anti-aggregants.
What about prevention?
One of the identified predictors for LAISA is DES implantation in ST-segment elevation infarction. Less endothelialisation of DES implanted in this context has also been observed due to strut penetration into a necrotic core46. This would entail a greater risk of late thrombosis in infarction, which has been reported in some registries but not in others and not in trials, although follow-up in these trials is only for two years.
Some have suggested performing a cutaneous hypersensitivity test before implanting a sirolimus-eluting stent.
Conclusions
LAISA is a relatively frequent finding (10-12%) with 1st generation DES, more so than with BMS. The aetiological mechanism is principally regional positive remodelling and thrombus lysis under the stent may contribute to this. It seems to occur more frequently in DES implantation in infarction. No data exists about LAISA in DES implanted in vein grafts.
LAISA after DES implantation, in its most extreme forms, is associated with very late thrombosis, which is an infrequent clinical event. However only 1.5-2% of cases with incidental LAISA will present thrombosis in 2-3 years .
It is not clear whether this represents a primary thrombosis-favouring mechanism or whether it is a marker of other more thrombogenic factors, such as decreased endothelialisation.
There is no evidence regarding the stance to be taken in cases where this finding, which is almost always incidental, is observed. The potential benefits and risks of LAISA correction are completely unknown. Only to extend combined antiplatelet therapy could be recommended.
It is necessary to consider that 2nd generation DES, included the new ones with bioabsorbable polymers, may show a lower incidence of LAISA, although information is much scarcer and is only available from a few controlled trials.

