- drug-eluting stent
- creatine phosphokinase-MB
- percutaneous coronary intervention
Abstract
Aims: The relationship between stent design and periprocedural myocardial infarction is unclear. The newest type of drug-eluting stent (DES), the everolimus-eluting stent (EES), has thinner strut and polymer thickness and a small amount of drug. EES use, therefore, could be related to less myocardial necrosis post-percutaneous coronary intervention (PCI). This study was undertaken to compare the amount of myocardial necrosis associated with implantation of the four DES types currently approved in the U.S.
Methods and results: A cohort of 7,259 consecutive patients who underwent elective PCI with DES implantation from 2003 to 2010 was studied. Sirolimus-eluting stents were deployed in 4420, paclitaxel-eluting stents in 1858, zotarolimus-eluting stents in 308, and EES in 673. The maximum creatine phosphokinase-MB (CPK-MB) was measured post-procedure and served as the primary endpoint. Patients who received EES had the smallest CPK-MB rise (p<0.0001) among the four groups. Further, a post-PCI increase was less often large enough to meet any of the usual criteria for periprocedural myocardial infarction used in clinical studies. After adjustment for baseline and angiographic characteristics, paclitaxel-, sirolimus-, and zotarolimus-eluting stent groups more frequently exceeded the CPK-MB >5x normal limit compared with EES-treated patients (OR=2.44, p=0.01; OR=2.01, p=0.05; OR=1.80, p=0.2, respectively).
Conclusions: It is plausible that EES with their superior design are associated with less periprocedural myocardial injury compared with older-generation DES.
Introduction
An increase in serum creatine phosphokinase-MB (CPK-MB) level after percutaneous coronary intervention (PCI) predicts an adverse late outcome1-5. Moreover, it has been consistently reported that the greater the rise, the more likely it is that such late events will occur1-5. In a meta-analysis of seven studies that included over 23,000 patients, Ioannidis et al have shown that 5x the upper limit of normal (ULN) is a better predictor of long-term mortality5. Certain pre-procedural clinical and angiographic characteristics, as well as procedural complications, are associated with the amount of post-PCI CPK-MB increase1-7. However, the relationship between stent design and periprocedural CPK-MB release is still unclear and has not been well studied with either bare metal stents or drug-eluting stents (DES). The design of the everolimus-eluting stent (EES) (Xience V; Abbott Vascular, Santa Clara, CA, USA / Promus; Boston Scientific, Natick, MA, USA) differs from that of other DES, with very thin struts and polymer as well as lower drug load. The randomised SPIRIT III: A Clinical Evaluation of the Investigational Device XIENCE V® Everolimus Eluting Coronary Stent System in the Treatment of Subjects With de Novo Native Coronary Artery Lesions trial reported that the use of EES was associated with less periprocedural myocardial infarction when compared to the paclitaxel-eluting stent (Taxus Express; Boston Scientific, Natick, MA, USA)8. However, it is unknown whether these results are applicable to routine clinical practice. This study was undertaken to compare the amount of myocardial necrosis associated with the implantation of the four, currently approved DES in the USA.
Methods
Patients
An ongoing registry of patients undergoing PCI at Washington Hospital Centre is maintained. From April 2003 to February 2010 there were 21,209 patients in our registry. After excluding those with ST-elevation myocardial infarction and non-ST-elevation myocardial infarction, a pre-procedure elevation in CPK-MB, or those without a DES, we reviewed the 8,617 patients who underwent elective PCI with DES. All had given written informed consent for the PCI procedure, and the study was conducted with the approval of the Institutional Review Board for the MedStar Health Research Institute.
Stent use
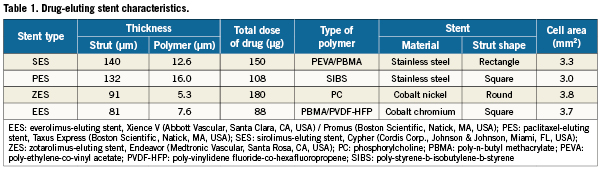
All 8,617 patients received at ≥1 DES. In order to focus the analysis on the particular type of DES deployed, we further excluded 1,358 patients for the following reasons: 1) they received a bare metal stent in addition to the DES, n=1000; 2) they received >1 type of DES, n=191; 3) they did not have a successful PCI, n=167; or 4) the value of CPK-MB were not available, n=46. Thus, the study cohort consisted of 7,259 patients all treated exclusively with one of the four DES varieties. Of them, 4,420 received sirolimus-eluting stents (Cypher; Cordis Corp., Johnson & Johnson, Miami, FL, USA), 1,858 received paclitaxel-eluting stents, 308 received zotarolimus-eluting stents (Endeavor; Medtronic Vascular, Santa Rosa, CA, USA), and 673 received EES (Table 1). The stent selection and the interventional strategy were left to the discretion of the physician.
Data collection
CPK-MB is routinely drawn on every patient undergoing PCI. Demographic, clinical, and procedural data, along with in-hospital outcomes, were collected and entered into a prospective database. Data were obtained from hospital chart review by independent research personnel blinded to the study objectives. All data management and analyses were performed by a dedicated data coordinating centre (Data Center, Cardiovascular Research Institute, Washington, DC, USA). Clinical outcome was obtained by trained quality assurance nurses who worked exclusively with the database to determine post-PCI clinical events. A committee independently adjudicated the nature of all clinical events.
Clinical endpoints and definitions
The maximum post-procedure CPK-MB level was chosen as the estimate of the degree of myocardial injury. CPK-MB was routinely measured before and immediately after the procedure and then every eight hours until a peak level was reached. In our laboratory 2.4ng/ml is considered the ULN. In this report we will compare the median peak level for all patients receiving each variety of stent. Further, to identify the frequency with which one of the several existing criteria for post-procedure myocardial necrosis was reached, we compared the proportion of patients receiving each stent type whose peak CPK-MB exceeded: the ULN, 2x ULN, 3x ULN, 4x ULN, and 5x ULN. We chose 5x ULN for our multivariable analysis5.
Secondary endpoints included Q-wave myocardial infarction, defined as the association of a biomarker rise of ≥2x ULN and the appearance of new Q-waves in the electrocardiogram in ≥2 contiguous leads. The complete definitions of all variables and the angiographic adverse events are available at the American College of Cardiology (ACC) website: (http://www.accncdr.com/WebNCDR/NCDR Documents/datadictdefsonlyv30.pdf) Target lesion complexity was denoted according to the ACC/American Heart Association (AHA) lesion classification system. Angiographic success was defined as a residual stenosis <30% with Thrombolysis In Myocardial Infarction 3 flow.
Statistical analysis
Normally distributed, continuous variables are expressed as mean ±standard deviation, and compared using an analysis of variance. For variables not normally distributed, median and interquartile ranges are reported. For categorical variables proportions and percentages are noted. The differences among groups were tested using the Chi-square test or Fisher’s exact test for categorical variables. The significant variables were compared using analysis of variance with post hoc analysis using Bonferroni correction. Because maximum CPK-MB was not normally distributed, the data were analysed using Kruskal-Wallis rank test.
The association between the endpoint and baseline variables was assessed by univariable and multivariable logistic regression analysis. All variables in Tables 2 and 3 and the year of enrolment were entered into the univariable analysis. The EES was treated as a reference for comparison of DES. Variables with a p value <0.1 on univariable analysis were entered into the multivariable logistic regression to adjust for baseline differences. All variables were entered into the model in their original form without transformation. Statistical analysis was performed using SAS version 9.1 (SAS Institute, Cary, NC, USA). Statistical significance was accepted for all values of p<0.05 except for post hoc analysis when <0.017 (0.05 divided by 3) was required for significance.
Results
Baseline characteristics
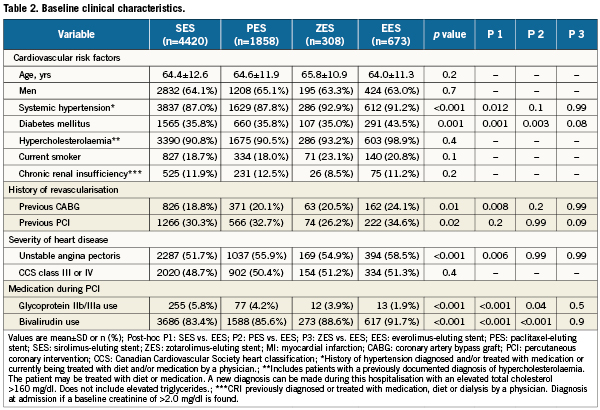
Table 2 compares patients’ baseline clinical characteristics. For the characteristics in which a statistical difference was found, a direct comparison with EES patients was undertaken. Columns P1 to P3 depict that comparison. The frequency of diabetes mellitus was significantly greater in EES patients than in sirolimus-eluting stent and paclitaxel-eluting stent patients. Further, systemic hypertension, unstable angina pectoris and history of coronary artery bypass graft surgery were more frequent in EES patients when compared with sirolimus-eluting stent treated patients. The rate of glycoprotein IIb/IIIa use was ≤5.8% in all four stent groups. As is the practice in this laboratory, use of bivalirudin in lieu of heparin for intra-procedural anticoagulation was high in all four groups.
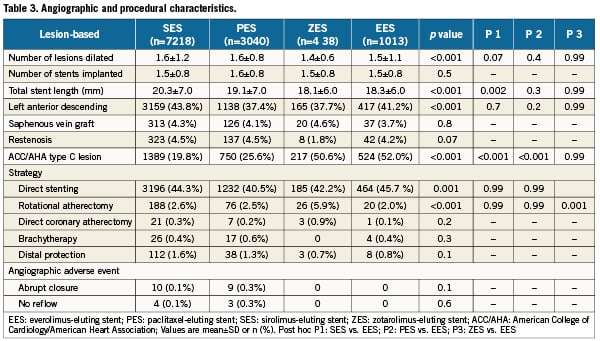
Table 3 depicts the baseline angiographic and procedural characteristics based on a by-lesion analysis. The mean length of stents deployed in EES-treated patients was slightly less than in those treated with sirolimus-eluting stents. Direct stenting was the strategy of choice in about 45% of all four patient groups and use of adjunctive devices (i.e., rotational atherectomy) was very low across all groups. Notably, EES and zotarolimus-eluting stents were chosen nearly twice as frequently in ACC/AHA type C target lesions than in the two other stent designs. In our population which consisted of those with successful PCI, abrupt re-closure and no reflow were rare.
In-hospital outcome
Table 4 provides a comparison of in-hospital outcomes. The rates of in-hospital death and Q-wave myocardial infarction were ≤0.6% for each of the DES cohorts. The median peak post-procedure CPK-MB was lowest for patients in whom an EES was deployed (p<0.0001). The proportion of patients in each stent cohort in which the CPK-MB increase following DES implantation exceeded five strata of the ULN is depicted in Figure 1. A smaller proportion of patients with EES placement exceeded the upper limit at each threshold.
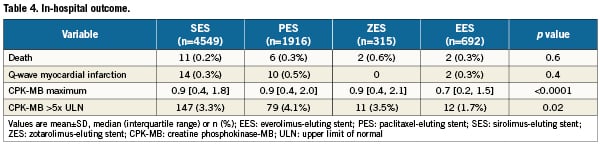
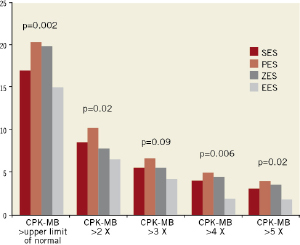
Figure 1. Creatine phosphokinase-MB (CPK-MB) maximum post elective percutaneous coronary intervention (PCI). CPK-MB maximum post-elective PCI of patients with sirolimus-eluting stents (SES), paclitaxel-eluting stents (PES), zotarolimus-eluting stents (ZES) and everolimus-eluting stents (EES) is shown. EES group had significantly lower CPK-MB maximum than any other groups. PES group had the highest CPK-MB maximum among stent groups for each endpoint.
Multivariable adjustment
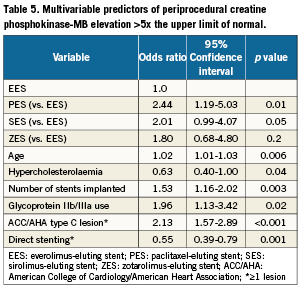
The year of enrolment was not associated with peak CPK-MB exceeding 5x ULN in the univariable model (p=0.23). Multivariable adjustment of this outcome (Table 5) demonstrated that when compared to patients receiving EES, patients treated with a paclitaxel-eluting stent were 140% more likely to have a CPK-MB increase >5x ULN (odds ratio 2.44, 95% confidence interval: 1.19-5.03, p=0.01). Moreover, a greater number of sirolimus-eluting stent-treated patients exceeded the >5x ULN threshold but the difference was only marginally significant (p=0.05). Other important predictors of a 5x ULN increase in CPK-MB included age (p=0.006), treatment of complex (ACC/AHA type C) lesions (p<0.001), and number of stents implanted (p=0.003). Also noteworthy was the finding that the incidence of an increase of this magnitude in CPK-MB was about 50% less in patients treated with direct stenting.
Discussion
Our data demonstrate that the amount of post-PCI CPK-MB increase is influenced by the type of DES deployed. The maximum CPK-MB level after EES placement was less than that reported after deployment of any of the other three DES. (Table 4, p<0.0001) Further, the maximum level in the EES cohort was more often lower than that in the other three stent cohorts with regard to the usually employed thresholds for the diagnosis of post-PCI infarction (Figure 1). Even after adjusting for potential confounders, the incidence was significantly lower in the EES cohort than in patients receiving paclitaxel-eluting stents (Table 5). Thus, it is plausible that patients who receive EES have less periprocedural myocardial necrosis than that encountered in the other three stents. This study therefore confirms the finding of Spirit III8, a randomised comparison of EES and paclitaxel-eluting stents. In that trial, more periprocedural myocardial infarctions were noted in patients with paclitaxel-eluting stents.
It is to be expected, however, that less myocardial injury at the time of PCI would translate into improved outcomes. No differences were seen in in-hospital death and Q-wave myocardial infarction, but previous work has often demonstrated an adverse effect on late outcomes of a CPK-MB rise post PCI1-5. Importantly, Tardiff et al1 found that elevated levels of cardiac biomarkers, whether or not associated with PCI and regardless of magnitude, should be interpreted as a myocardial infarction.1 It follows that the adverse influence on intermediate- and long-term prognoses associated with non-Q-wave myocardial infarction attaches to periprocedural CPK-MB elevation.
Previous studies
Previous studies comparing long-term follow-up in first and second generation DES have found improved safety and efficacy for the latter8,9. Since periprocedural myocardial necrosis is associated with adverse outcomes1-5, it is reasonable to conclude that the benefits associated with the newest generation of DES is in part attributable to reduced myocardial injury. EES remarkably decreased the amount of rise in periprocedural CPK-MB when compared with each of the older-generation DES (Figure1). On the other hand, our data do not show the same beneficial effect associated with zotarolimus-eluting stent use. In fact, although the difference was not statistically significant (0.2), we observed an 80% greater likelihood that a rise of CPK-MB >5x ULN would follow zotarolimus-eluting stent use as compared with the use of an EES (Table 5).
Reasons for EES advantage
Intravascular injury from stent placement and downstream microvascular embolisation of atheromatous and thrombotic particulate debris almost surely accounts for myocardial injury associated with PCI and the attendant CPK-MB rise7,10,11. The EES consists of thin-strut, cobalt-chromium frame with a thin coating of a biocompatible fluoro-polymer on an open cell, mounted on a semi-compliant balloon. Such stent design can produce less vascular injury and thereby less myocardial necrosis. The zotarolimus-eluting stent (ZES) has a round shape strut, while the EES has a square shape strut. This difference could be one of the reasons why EES was superior to ZES in terms of periprocedural myocardial necrosis. It is also possible that differences in the strut metal used or differences in drug type and/or its dose account for the observed differences, but the mechanism for this possibility is less obvious than is the case with the mechanical advantages of a less bulky stent, which consists of thin strut and polymer and large cell area. In favour of deploying less stent metal, our observations show that the number of stents implanted is an independent predictor of periprocedural CPK-MB increase7,12.
Other contributors to risk of CPK-MB release
Older age is also an independent predictor for periprocedural CPK-MB increase (p=0.006). Others have reported that plaques in elder patients contain more necrotic core when compared to plaques in younger patients13 and could therefore have a greater risk of embolisation. On the other hand, direct stenting, arguably less traumatic for the vascular wall, was in this data set significantly protective for periprocedural CPK-MB increase (p=0.001). Others have reported that direct stenting of DES is feasible and highly successful14.
In our institution, bivalirudin rather than heparin is routinely used for nearly all cases. That being so, glycoprotein IIb/IIIa inhibitors are only used for particularly complex lesions and procedural complications. Thus, any protective effect such agents may provide is overshadowed by other factors. Treating saphenous vein grafts carries a high risk of distal embolism, but in this data set stenting of such lesions was not independently associated with the amount of CPK-MB release. It is plausible that operators, recognising the embolic risk associated with treating such lesions, successfully reduced the risk by using direct stenting15, distal protection16, or undersized stents17 in DES era.
Limitations
Because of the observational nature of the study, unmeasured potential confounders are unavoidable. For example, some detailed angiographic (e.g., number of bifurcation lesions treated and number of target lesions containing thrombus) and procedural information (e.g., inflation pressure of dilating balloon, frequency of side branch occlusions), are lacking. Furthermore, consistent information regarding preadmission medication is also missing. Chronic statin or beta-blocking agents could protect for CPK-MB increase18. It is possible that the lack of statistically significant differences between EES and zotarolimus-eluting stents is due to the relatively small number of zotarolimus-eluting stents. The usage distribution of stent types over time is not equal and this could influence the results even after adjustment. Lastly, it could be argued that post-PCI troponin increase may provide a better marker of myocardial injury. The information on that biomarker is not consistently available. Nonetheless, the value of our data rests on the fact that it is the only study with a large cohort to examine the relationship between different DES types and the amount of myocardial necrosis associated with each.
Conclusion
The type of DES significantly affects the amount of CPK-MB increase post-PCI. Of the four stent types investigated, patients with EES had less periprocedural CPK-MB increase than did patients receiving one of the other three types. It may be that the EES was designed to have the smallest dose of drug, the thinnest struts with large cell area of any of the four. It can be argued that the amount of vascular injury is smallest in that stent and that consequently the amount of distal embolisation is smallest.
Conflict of interest statement
The authors have no conflicts of interest to declare.

