Abstract
Background: The BioFreedom drug-coated stent with a stainless steel platform (BF-SS) has been demonstrated to be efficacious in patients at high bleeding risk and receiv-ing only one-month dual antiplatelet therapy.
Aims: The aim of this study was to evaluate the efficacy of the new BioFreedom Ultra drug-coated stent with a thin-strut cobalt-chromium platform (BF-CoCr) compared to the BF-SS in an all-comers population undergoing percutaneous coronary intervention (PCI).
Methods: This was a prospective, multicentre, non-inferiority trial. The primary endpoint was in-stent late lumen loss (LLL) as determined by quantitative coronary angiography at nine-month follow-up. Clinical evaluation was performed at one year.
Results: A total of 200 patients were randomised (1:1) to either the BF-CoCr or the BF-SS stent at eight centres in Spain and Denmark. Baseline clinical and lesion characteristics were similar between the groups. Mean age was 66 years and 23% were female. The mean number of stents implanted per patient was 1.5. At nine-month follow-up, mean in-stent LLL was 0.34±0.49 mm in the BF-CoCr group versus 0.29±0.37 mm in the BF-SS group, p=0.005 for non-inferiority. At one year, target lesion failure was similar between the groups (7.3% in BF-CoCr vs 9.3% in the BF-SS group; p=0.60).
Conclusions: The BF-CoCr was non-inferior to the BF-SS in terms of in-stent LLL at nine months. Larger studies powered for clinical endpoints are warranted to compare the efficacy of this new platform with currently available DES.
Introduction
The antirestenotic efficacy of drug-eluting stents (DES) has been demonstrated in large randomised trials. This has led to their widespread use in daily practice, making them the treatment of choice for patients undergoing percutaneous coronary intervention (PCI) in any clinical setting1,2,3,4. Since the approval of first-generation DES, stent design and several technical aspects have evolved, including the use of thinner stent struts, biodegradable polymers and the preferential use of cobalt-chromium (CoCr)-based alloys for the stent platform. The polymer-free biolimus-coated stent was originally developed to minimise the potential long-term adverse effects associated with polymer coatings. The first iteration had a stainless steel (SS) platform with a strut thickness of 112-120 µm and a micro-structured abluminal surface to optimise drug delivery. This enabled drug-to-vessel wall tissue transfer from the stent to be complete within 28 days of treatment leaving the implant behind as a bare metal stent. This rapid drug transfer to the vessel wall provided a rationale for an abbreviated dual antiplatelet therapy, which was an attractive treatment option for patients at high bleeding risk. The LEADERS FREE trial demonstrated the superior efficacy and safety of the BioFreedom™ SS (BF-SS; Biosensors Europe, Morges, Switzerland) platform compared to the bare metal comparator stent in patients at high bleeding risk and receiving only one-month dual antiplatelet therapy following stent implantation5.
Since then, a second iteration of the BioFreedom device has been introduced with a thin-strut (84-88 µm) Co-Cr platform (BF-CoCr; Biosensors Europe) to improve the performance of the device further. This new platform allowed a reduction of the stent strut thickness while maintaining similar radial strength. Other design elements including the micro-structured abluminal surface, the Biolimus A9 drug, the drug dose and release kinetics are all identical to those of the previous BF-SS stent.
These features may provide the new iteration with additional advantages in terms of acute performance and antirestenotic efficacy. Therefore, we designed the BioFreedom QCA randomised clinical trial (ClinicalTrials.gov Identifier: NCT03307213) to evaluate the antirestenotic efficacy of the new BF-CoCr drug-coated stent in an all-comers population as compared to the first-generation BF-SS stent.
Methods
STUDY POPULATION
This trial enrolled adult patients with symptomatic coronary artery disease including chronic and acute coronary syndromes (ST-segment and non-ST-segment elevation myocardial infarction) who had an indication for PCI. As an angiographic inclusion criterion, the target lesion size should range between 2.5 and 3.5 mm to be covered by the available stent sizes. No other limitations on the number of lesions or vessels to be treated or lesion length were imposed. There were major exclusion criteria (Supplementary Appendix 1).
STUDY DESIGN AND RANDOMISATION
The BioFreedom QCA trial was a prospective, multicentre, open-label, randomised study that compared the performance of the BF-SS versus the BF-CoCr stent in an all-comers population presenting with the full spectrum of coronary artery disease. Once the patient signed the informed consent and the above criteria were met, randomisation (1:1) was performed through an interactive web recognition system using random permuted blocks within strata of sizes 4 and 6 to receive either the BF-SS or the BF-CoCr stent. The randomisation schedule was computer generated and stratified by the presence or absence of diabetes mellitus.
PROCEDURES
PCI with the allocated stent was performed according to the local standard of care. Treatment of multiple target vessels (within the same procedure) and staged procedures within six weeks of the initial index procedure were permitted with the use of the assigned stent type as per randomisation. Therefore, any subsequent treatment of a lesion that was already present (but was not treated) at the time of the index procedure was considered as a staged procedure. Dual antiplatelet therapy was prescribed according to current clinical guideline recommendations.
ENDPOINTS
The primary endpoint of the study was in-stent late lumen loss (LLL) assessed by quantitative coronary angiography (QCA) at nine months. Secondary endpoints at all follow-up time points included all-cause and cardiac death, non-fatal myocardial infarction (MI), clinically indicated target lesion and target vessel revascularisation (CI-TLR; CI-TVR), major adverse cardiac events (MACE, defined as the composite of cardiac death, MI or CI-TLR), target lesion failure (TLF, defined as the composite of cardiac death, target vessel-related MI, CI-TLR), stent thrombosis per ARC definition6, device success, procedure success, and lesion success. Definitions of these endpoints are available in Supplementary Appendix 1.
FOLLOW-UP
Patients were scheduled to be followed after hospital discharge at 30 days, 9, 12 and 24 months. In addition, at 9 months post index procedure, another angiography was performed for QCA analysis.
QCA ANALYSIS
Core lab QCA assessments (HCor, Sao Paolo, Brazil) were performed at baseline, post procedure and after the 9-month follow-up angiography to assess the primary endpoint. Additionally, the core lab assessed all cases of stent thrombosis and revascularisation. A technical description of the angiographic assessment is available in Supplementary Appendix 1.
STUDY COMMITTEES
Members of the Data Safety Monitoring Board and of the Clinical Events Committee are shown in Supplementary Appendix 1.
SAMPLE SIZE CALCULATION
The hypothesis of the study was that the BF-CoCr was non-inferior to the BF-SS with respect to in-stent LLL. Assuming true equivalence of the means between both stents, with a common standard deviation (SD) of 0.45 mm and a non-inferiority margin of 0.20 mm, 160 evaluable patients were needed in order to yield 80% power for non-inferiority using a one-sided, two-sample t-test with an alpha of 0.025. With an anticipated drop-out rate of 20%, we planned to enrol 200 patients. Statistical analysis and ethical considerations are detailed in Supplementary Appendix 1.
Results
BASELINE AND PROCEDURAL CHARACTERISTICS
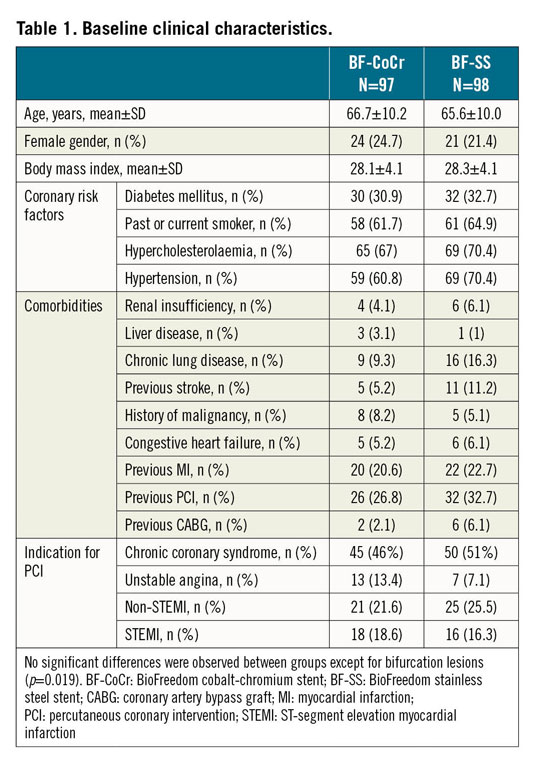
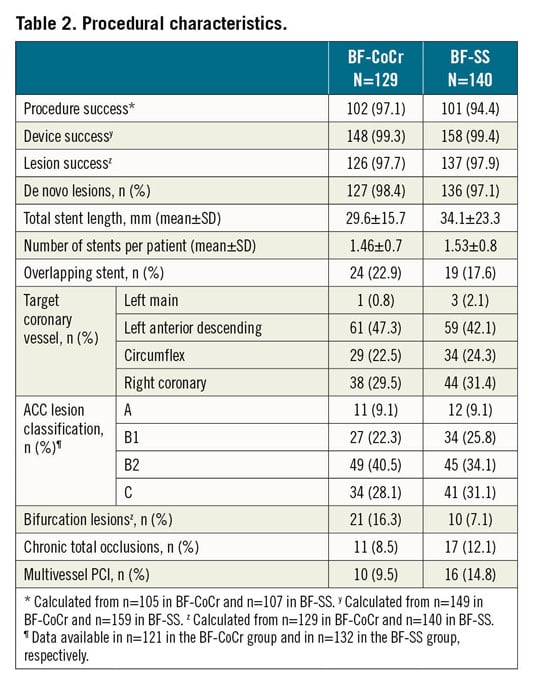
Between June 2018 and March 2019, 200 patients were randomised in eight centres in Denmark and Spain. In five patients PCI was not performed. Therefore, the final sample size was 195 patients with 211 treated lesions (modified intention-to-treat [mITT] population). The institutions involved are presented in Supplementary Appendix 1. The flow chart of the trial is shown in Figure 1. Baseline demographics, clinical and procedural characteristics are presented in Table 1 and Table 2. No major clinically relevant differences were observed between groups. The mean age was 66 years and 23% were female. Diabetes mellitus was present in one third of the patients and acute coronary syndromes in 40% of the recruited individuals. In terms of periprocedural variables, treatment of bifurcation lesions was significantly more often performed in the BF-CoCr group (16.3% vs 7.1% in the BF-SS arm; p=0.019).
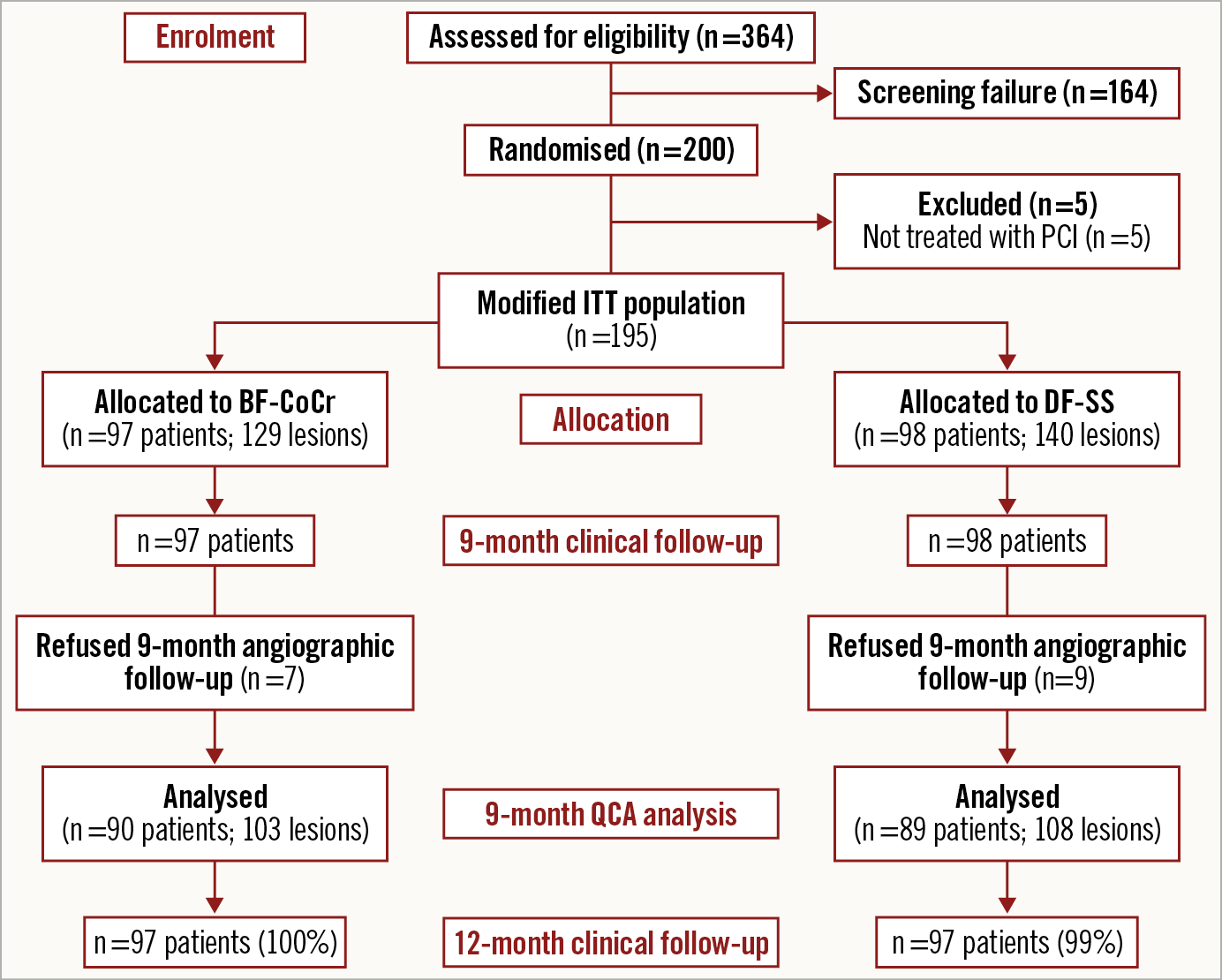
Figure 1. Study flow chart.
PRIMARY ENDPOINT ANALYSIS
Baseline and post-procedure QCA data were similar between the groups (Table 3). Follow-up angiography was performed in 90 patients (103 lesions) in the BF-CoCr group (92.8% of those allocated) and in 89 patients (108 lesions) in the BF-SS group (90.8%) (Figure 1). Mean in-stent LLL was 0.34±0.49 mm in the BF-CoCr group versus 0.29±0.37 mm in the BF-SS group (p for non-inferiority=0.005). The per-protocol population yielded similar results with non-inferiority also reached. The cumulative frequency distribution curve for LLL of the two stent types is displayed in Figure 2. Mean LLL was similar between diabetic and non-diabetic patients (0.28±0.29 mm and 0.33±0.45 mm, p=0.23). Also, there were no differences between stent types both in diabetics and in non-diabetics (0.33±0.44 mm in BF-CoCr vs 0.24±0.35 mm in BF-SS, p=0.353, and 0.34±0.52 mm in BF-CoCr and 0.31±0.35 mm in BF-SS, p=0.745, respectively).
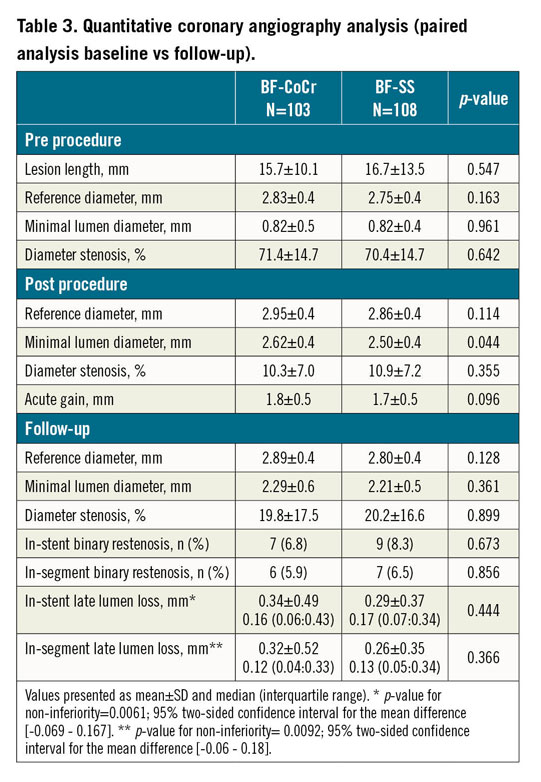
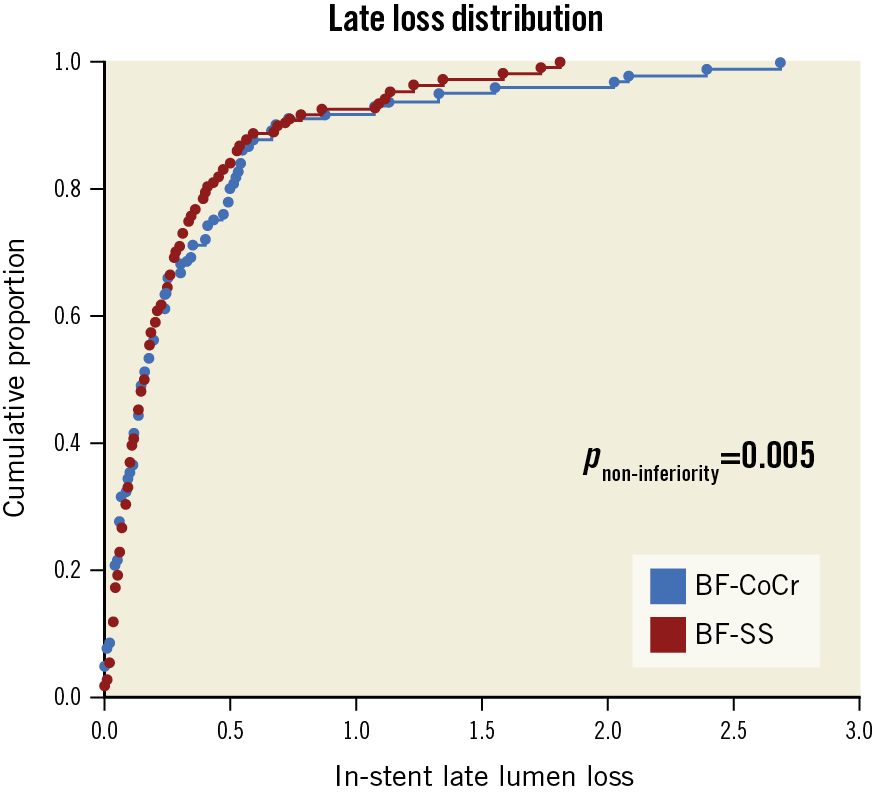
Figure 2. Cumulative distribution curves of the LLL between the BF-CoCr and BF-SS groups. LLL: late lumen loss
CLINICAL FOLLOW-UP
Rates of procedure, lesion, and device success were comparable between groups (Table 2). At 12 months, clinical events could be obtained in all patients included (Figure 1); no significant differences were apparent between the two stent groups (Supplementary Table 1). The antithrombotic therapy regimen up to 12 months is presented in Supplementary Table 2 and Supplementary Table 3. Overall, at 12 months, the proportion of patients on dual antiplatelet therapy was 59.2%, with similar proportions in both arms (64.6% in the BF-SS arm vs 53.7% in the BF-CoCr arm; p=0.125). A total of 17.3% of patients received oral anticoagulation therapy without differences between groups. In patients with acute coronary syndromes on admission, the rate of dual antiplatelet therapy at 12 months was higher (67.1%) than that of patients with chronic coronary syndromes (53.6%), with no differences between groups.
Discussion
This study represents the first-in-human clinical experience of the new BF-CoCr stent in an all-comers cohort of patients presenting with the entire spectrum of coronary artery disease. This new platform demonstrated non-inferiority for LLL at nine-month angiographic follow-up compared with its market-approved precursor, the BF-SS. The main difference between the two stents is the cobalt-chromium alloy that allowed a reduction in strut thickness from 120 µm of the BF-SS to approximately 84-88 µm for this new platform. All other design elements, including the polymer-free design, the antiproliferative drug, the dose, the release kinetics and the achieved target tissue drug concentrations, were not different between the stents.
IN-STENT LATE LUMINAL LOSS AS SURROGATE ENDPOINT
In-stent LLL is calculated as the difference in minimal luminal diameter inside the boundaries of the stent between that achieved post index procedure and that observed at angiographic follow-up. This parameter has been widely used to assess the antirestenotic efficacy of various angioplasty techniques such as balloon angioplasty, atherectomy, intracoronary brachytherapy, bare metal stents, DES, and bioresorbable scaffolds, among others1,2,3,4,7,8,9,10,11. As a continuous variable, the required sample size for a trial would be smaller than that required for a binary angiographic parameter such as restenosis rate or a clinical parameter such as TLR. Consequently, LLL has been routinely used as the reference standard for stent efficacy comparisons and for device approval by regulatory bodies. In the bare metal stent era, restenosis was the major limitation for stenting, with values of LLL commonly ranging between 0.8 and 1.2 mm12. With the advent of first-generation DES, neointimal proliferation was dramatically suppressed with a subsequent reduction in LLL values to <0.2 mm1. Although these low values of LLL were initially associated with negligible rates of TLR, safety concerns in terms of stent thrombosis or late restenotic catch-up phenomenon started to appear in the long term13. Consequently, the lower the better concept as it referred to LLL became debatable. Clearly, LLL has good discriminating capability for clinical outcomes in patients treated with devices or techniques with rather poor antirestenotic efficacy14. However, below a certain threshold, this parameter may lose the ability to predict the occurrence of clinical events such as TLR. Other vascular factors such as completeness of the healing process, the occurrence of late acquired stent malapposition or inflammatory and hypersensitivity reactions15 may be more relevant for a patient’s long-term outcomes than the angiographic quantification of their lumen loss.
CLINICAL CORRELATES OF LATE LUMINAL LOSS
In data from a pooled analysis of trials using bare metal and first-generation DES, Pocock et al demonstrated an exponential relationship between LLL and TLR, suggesting that low values for LLL were not associated with an appreciably increased incidence of TLR at one year16. Recently, Asano et al investigated the relationship between LLL and clinical outcomes with newer-generation DES. In a patient-level meta-analysis of seven trials (2,426 patients) and study-level meta-analysis involving 40 trials (19,199 patients), the exponential relationship between in-stent LLL and the incidence of TLR was confirmed with an optimal cut-off value of LLL for a TLR event of 0.50 mm17. The authors suggested that this cut-off value could be used as the upper limit non-inferiority boundary of LLL when objective performance criteria are used for device efficacy assessment.
In terms of safety, a mild or moderately increased LLL might be favourable regarding completeness of stent coverage. Indeed, a very low LLL may reflect a delayed and incomplete healing process with uncovered and malapposed struts, only seen on optical coherence tomography18.
Mean values of LLL evidenced in this trial for both arms were well below the 0.5 mm threshold but higher than other currently available DES that typically present values <0.20 mm. Variations in LLL values across trials can be related to variability of core lab analyses and different timing of the angiographic follow-up. Interestingly, a broad SD and non-normal distribution of LLL are typically seen with DES8. As such, comparison of medians rather than means could be more accurate. In this regard, both the BF-CoCr and BF-SS showed median values of LLL in the range of 0.16-0.17 mm (Table 2).
CLINICAL RELEVANCE OF STRUT THICKNESS
The importance of the strut thickness was demonstrated in the bare metal stent era. Kastrati et al compared the angiographic performance of otherwise identical ultra-thin (50 µm) versus thick-strut (140 µm) bare metal stents manufactured by the same company. Rates of angiographic restenosis and LLL were significantly lower in the thin-strut device group (15% vs 25.6%; p=0.003, and 0.94±0.74 mm vs 1.17±0.78 mm, p=0.001; thin-strut vs thick-strut, respectively)12. However, in the DES era, the importance of strut thickness to prevent restenosis may be less relevant. As mentioned above, the antirestenotic efficacy of the first-generation sirolimus-eluting stent (CYPHER®; Cordis, Cardinal Health, Milpitas, CA, USA) with a 140 µm strut thickness was the highest (LLL nearly 0 mm) among other comparable first-generation DES – the paclitaxel-eluting DES TAXUS™ Liberté™ (Boston Scientific, Marlborough, MA, USA) with 96 µm and LLL around 0.40 mm; the zotarolimus-eluting DES Endeavor® (Medtronic, Minneapolis, MN, USA) with 91 µm and LLL around 0.60 mm19,20. Thick rectangular struts may be associated with stent restenosis and thrombogenicity through creating areas of recirculation with low endothelial shear stress that increase local concentration of activated platelets, retard re-endothelialisation, and attenuate the production of natural anticoagulants21. Therefore, the development of new platform alloys for current-generation DES capable of reducing strut thickness may be more influential in the device’s acute performance in complex anatomical scenarios than in their ability to reduce TLR per se22. The latter may probably be inherent to the biocompatibility of the device coating, type of antirestenotic drug and release kinetics. In addition, the clinical benefit of thin-strut devices may also derive from a long-term reduction of the rates of stent thrombosis and myocardial infarction23.
Limitations
The major limitation of the study is the small sample size that does not provide sufficient power to confirm non-inferiority for the clinical endpoints. However, this is a typical feature of clinical studies powered for surrogate endpoints. Secondly, the study was not designed to use intracoronary imaging techniques to assess the healing process of both stent types. In addition, a non-inferiority margin of 0.20 mm represents 44% of the SD. This might represent a potential limitation of the trial. However, this margin has been chosen in similar head-to-head trials. Longer follow-up is needed to confirm the safety profile of this new platform after discontinuation of dual antiplatelet therapy. Finally, we cannot infer the safety of this new platform for patients receiving only a one-month dual antiplatelet regimen.
Conclusions
In summary, this study documents non-inferiority for the new BF-CoCr stent in comparison with its precursor, the BF-SS stent, for the primary angiographic endpoint of in-stent LLL. Larger studies powered for clinical endpoints are warranted to compare the efficacy of this new platform with currently available DES.
|
Impact on daily practice The results of this first-in-human trial support the use of the new cobalt-chromium platform of the BioFreedom stent in patients with a wide spectrum of coronary artery disease. This new platform will improve the performance of the currently available stainless steel BioFreedom stent. |
Acknowledgements
The authors wish to thank all the patients, physicians, nurses and study coordinators from all recruiting centres for their commitment to the study.
Funding
The trial was sponsored by Biosensors Europe S.A.
Conflict of interest statement
M. Sabaté has received consultant fees from Abbott Vascular and iVascular outside the submitted work. A. Perez de Prado has received consultant fees from B. Braun, Boston Scientific and iVascular outside the submitted work. A. Cequier has received consultant fees from Abbott Vascular, Biotronik and Medtronic outside the submitted work. J. Flensted Lassen has received consultant fees from Abbott Vascular, Boston Scientific and Medtronic outside the submitted work. D. Schütte and H.-P. Stoll are employees of Biosensors. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

