Abstract
Aims: Bare metal stents continue to be used for the interventional treatment of coronary artery disease. We report the clinical and angiographic results of a multicentre, single-arm evaluation of safety and feasibility of the MOMO stent (Japan Stent Technology Co., Ltd, Okayama Research Park Incubation Centre, Okayama, Japan).
Methods and results: The MOMO stent is a novel thin-strut cobalt-chromium carbon-coated stent for the treatment of de novo coronary artery disease (CAD). In this prospective, non-randomised, single-arm study, 40 patients (stable and unstable angina) with single-vessel CAD were recruited into the study from three centres. Patients with lesions ≤15 mm in length and with a target vessel diameter of ≥3 mm were eligible. Angiographic follow-up was performed at six months. Quantitative coronary angiography (QCA) was used to measure acute gain and late luminal loss (LLL). Intravascular ultrasound (IVUS) was performed in 15 consecutive patients from two centres to assess the degree of neointimal proliferation within the stented segment at six-month follow-up. The MOMO stent performed well without any procedural complications with an acute procedure and technical success rate of 100%. Repeat revascularisation was performed in six patients (15%) during the six-month follow-up. Ischaemia-driven revascularisation was documented in three patients (7.5%). No myocardial infarction, stent thrombosis or cardiac death was observed. One non-cardiac death was reported secondary to lung cancer. Binary restenosis was 12.5% (n=5), and the LLL was 0.54±0.3 mm.
Conclusions: This first-in-man experience demonstrates proof of concept of the safety and feasibility of the MOMO cobalt-chromium carbon-coated stent for patients with single focal de novo lesions presenting with stable and unstable CAD.
Introduction
Bare metal stents continue to be used for the interventional treatment of coronary artery disease1-3. Whilst drug-eluting stents (DES) have been proven to reduce the incidence of restenosis when compared to bare metal stents, their benefit appears to be most marked in lesions with high risk for intimal proliferation and is less meaningful in focal lesions situated in larger arteries1,4-6. Despite the reduced restenosis rates associated with DES, the need for more prolonged dual antiplatelet therapy may lead to the choice of bare metal stents in selected patient groups7,8.
The design and engineering of stent platforms is constantly evolving with major advancements made over the years9-11. Newer-generation drug-eluting stents do not have an increased risk of stent thrombosis when compared to bare metal stents12. The results of novel bare metal stent platforms have to be evaluated. It appears that current bare metal stents with thin struts and open-cell designs achieve better acute and long-term outcomes, when compared to historic cohorts of first and second-generation stents13,14.
We present the first clinical experience with a novel bare metal stent engineered from a laser-cut cobalt-chromium alloy with a carbon coating. It has been developed with a view to excellent deliverability and a minimal proliferative response following implantation into native coronary arteries. We report on the first forty patients treated with the MOMO stent for symptomatic coronary disease.
Methods
THE MOMO STENT
The MOMO balloon-expandable coronary stent (Japan Stent Technology Co., Ltd, Okayama Research Park Incubation Centre, Okayama, Japan) is an L605 cobalt-chromium stent platform laser cut from a single tube with 70 µm strut thickness (Figure 1). The closed-cell design is based on uniform interconnections of monotype cells without sharp joints or welding points (Figure 2). The profile of the stent is 0.96 mm.
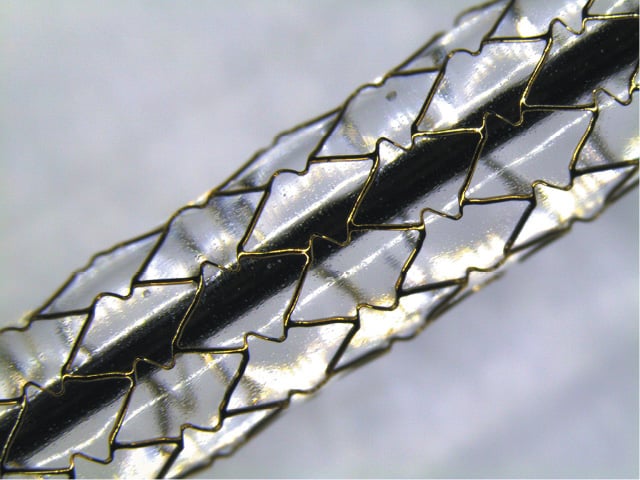
Figure 1. MOMO stent: 3.5 / 18 mm model on inflated delivery balloon.
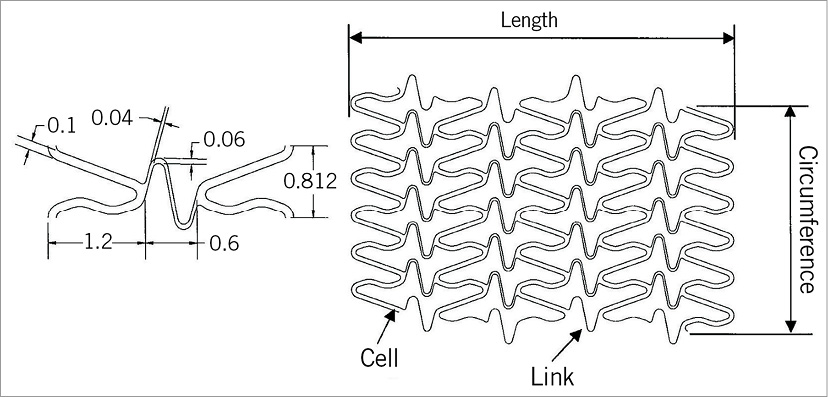
Figure 2. Schematic drawing of the stent structure.
The stent is coated with “diamond-like” carbon (DLC). The coating (average thickness: 35 nm) has the biocompatible and haemocompatible characteristics of pyrolitic carbon15,16. Nano-coating technology allows the DLC coating to track deformations when the stent is expanded, preventing disruption of the coating. The stent is premounted on the balloon between two platinum iridium radiopaque markers. The MOMO stent system is compatible with a 5 Fr outer diameter guiding catheter with a 0.058”/1.47 mm minimum inner diameter. For this study, the stent was available in 13 mm, 18 mm, and 23 mm lengths and 3.0 mm, 3.5 mm, and 4.0 mm diameters. The rapid exchange stent delivery system is based on a hypotube balloon catheter. In bench tests, once expanded to the desired diameter, the stent shows an average shortening of 2-5% and average recoil between 2.88% and 5.99% across the three sizes.
STUDY DESIGN
A multicentre, prospective, non-randomised, single-arm study was conducted at three participating centres (Bristol Heart Institute, Bristol, UK; St Marien-Hospital, Bonn, Germany, and St. Marien-Krankenhaus, Siegen, Germany). The primary objective was to assess the safety and feasibility of the MOMO stent in patients with de novo coronary artery disease. The study was approved by the local ethics committee at the participating centres and was conducted in accordance with the Declaration of Helsinki. Patient participation in the study was voluntary and the patients did not receive any financial remuneration during the course of the study. All patients provided a written informed consent.
PATIENT POPULATION
Patients were enrolled if they met all the inclusion and exclusion criteria. Patients were eligible for enrolment if they had stable or unstable angina, a single de novo lesion in a single native coronary artery with a target vessel diameter of ≥3 mm and a lesion length ≤15 mm.
The exclusion criteria related to angioplasty were as follows: multivessel, multilesion disease, in-stent restenosis, small vessel (<3.0 mm) disease, previous coronary artery bypass graft (CABG), bifurcation lesion, lesion length >15 mm, chronic total occlusion, severe calcification and angiographic evidence of thrombus. Patients with ST-elevation myocardial infarction, an ejection fraction less than 30%, left main stem coronary artery disease and ostial lesions were also excluded, as were patients with life expectancy of less than two years, contraindication to dual antiplatelet therapy and women with childbearing potential.
STENT IMPLANTATION
The patients were treated according to current daily practice in each of the participating centres. All patients received aspirin 300 mg and clopidogrel 600 mg therapy as a loading dose. A heparin bolus of minimum 5,000 IU IV was administered after sheath insertion. All lesions were predilated prior to stent implantation. Post-dilatation was left to the discretion of the operator. Dual antiplatelet therapy (aspirin 75 mg and clopidogrel 75 mg once daily) was continued for one month after the index procedure in patients with stable angina and for 12 months in patients with troponin positive acute coronary syndromes on presentation. After completion of the initial course of dual antiplatelet therapy, all patients were maintained on lifelong aspirin therapy.
DATA COLLECTION AND FOLLOW-UP
Clinical data were collected before and after the index procedure. A clinic visit was undertaken by all patients at 30 days and at six months. Telephone follow-up was conducted at three months.
All patients were scheduled for protocol-mandated six-month follow-up angiography. Intravascular ultrasound (IVUS) assessment was performed in consecutive patients at six months in only two (Bristol and Bonn) of the three centres. No IVUS images were obtained during the index procedure. Angiographic and IVUS images were analysed at the Bristol Heart Institute, Bristol, United Kingdom. Data were entered into a central database. All serious adverse events were adjudicated by an independent clinical events committee.
STUDY ENDPOINT AND DEFINITIONS
Technical success was defined as the ability to cross the lesion and deploy the MOMO stent at the target lesion. Device success was defined as technical success with the achievement of a final diameter stenosis of <30% and with thrombolysis in myocardial infarction (TIMI) 3 flow in the vessel on which the intervention was performed. Procedural success was defined as technical success without the occurrence of clinical complications (death, reinfarction, repeat revascularisation, access site complications, bleeding requiring blood transfusion, or cerebrovascular accident) during hospital stay.
The primary endpoint of the study was major adverse cardiac events (MACE) defined as the composite of cardiac death, recurrent myocardial infarction and repeat target vessel revascularisation (TVR) at six months. TVR was defined as a repeat revascularisation by either percutaneous coronary intervention (PCI) or CABG of the target vessel. Ischaemia-driven TVR (ID-TVR) was defined by the presence of objective evidence of ischaemia associated with recurrent angina or by a 70% diameter stenosis within the target vessel by QCA at follow-up angiography. Ischaemia-driven target lesion revascularisation (ID-TLR) was defined by the presence of objective evidence of ischaemia associated with recurrent angina or by a 70% diameter stenosis of the target lesion (stent+5 mm edges) by QCA at follow-up angiography. Death was defined by all-cause mortality including cardiac and non-cardiac causes.
Myocardial infarction (MI) was defined as the occurrence of clinical symptoms of chest pain associated with electrocardiographic abnormalities and new elevation of troponin above the upper limit of normal. Stent thrombosis (ST) was defined according to the Academic Research Consortium17.
ANGIOGRAPHIC ASSESSMENT
Quantitative coronary angiography (QCA) was performed using the Axiom Artis QCA software package (Siemens, Forchheim, Germany). The stented segment and the 5 mm peri-stent segments (proximal and distal to the stent) were analysed. Minimal luminal diameter (MLD), reference diameter (RD) and percentage diameter of stenosis were measured. Acute gain was defined by the difference between final MLD and baseline MLD. Binary restenosis was defined as a diameter stenosis of ≥50% at six-month follow-up. Late lumen loss was defined as the difference between MLD post procedure and six-month follow-up MLD.
IVUS ASSESSMENT
IVUS images were obtained using the AtlantisTM SR Pro system (Boston Scientific, Natick, MA, USA) with 40 MHz image acquisition, using a motorised pullback at a constant speed of 0.5 mm/second (Figure 3). A computer-based contour detection programme (iLab; Boston Scientific) analysed cross-sections of the lumen, stented section and the entire vessel to calculate the diameter, area and the volume. Stent volume (SV) and lumen volume (LV) were calculated by multiplying length by mean areas. The neointimal volume was defined as the difference between the SV and LV.
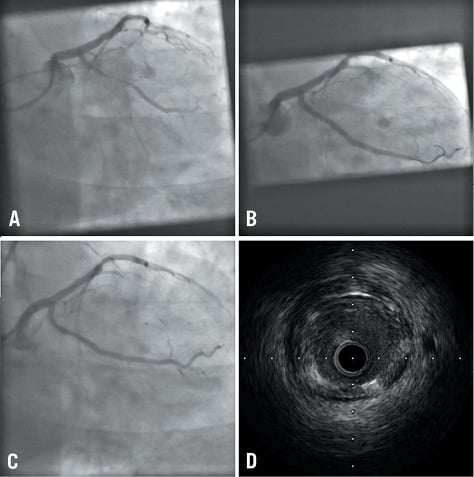
Figure 3. Case example. A) Pre stent deployment; B) Post stent deployment; C) Six-month follow-up; D) Intravascular ultrasound appearance at six months
Stent strut apposition was defined at follow-up as apposed (embedded), aligned (protruding) or incompletely apposed. Apposed struts were embedded within the vessel wall. Aligned struts were defined as struts with a distance between the vessel wall and the strut abluminal surface less than the thickness of the strut. Incomplete strut apposition (malapposition) was defined as clear separation between the strut and vessel wall with a distance greater than the thickness of the strut.
STATISTICAL ANALYSIS
This feasibility study was designed to provide preliminary observations about the safety and feasibility of the MOMO stent in humans. All analyses were performed according to the intention-to-treat principle. Variables are presented as numbers, percentages or mean (standard deviation values). All authors have read and agreed to the manuscript as written.
Results
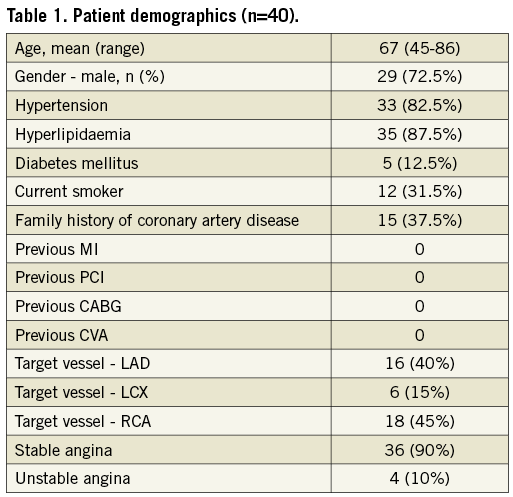
A total of 40 patients were enrolled. Table 1 shows the demographic characteristics of the study population and the location of the culprit lesion. The acute procedural and technical success rate was 100%. Four patients received two stents (one due to edge dissection and three due to suboptimal results at operator discretion) and one patient received three stents (suboptimal angiographic appearance). Post-dilatation was performed in 30 (75%) patients. No MACE occurred during the index hospitalisation. During the 30-day follow-up period, one patient died of lung cancer, diagnosed after the index procedure.
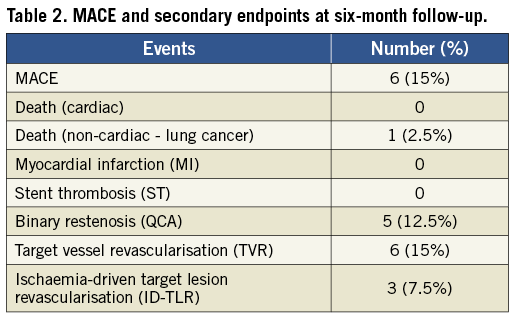
At six-month follow-up MACE was 15% (Table 2). There was no new MI, stent thrombosis or strokes. A total of six patients (15%) underwent revascularisation of the target vessel (including two of the three patients who did not undergo procedural post-stent dilation). Ischaemia-driven target vessel revascularisation (ID-TVR) occurred in ten (7.5%) patients. Two patients underwent repeat target lesion revascularisation with a drug-eluting stent (DES), two patients with TLR in the proximal left anterior descending artery (LAD) were referred for CABG and two patients underwent coronary balloon angioplasty. Only three of these patients met the criteria for ID-TVR. The non-ischaemia-driven revascularisations were performed on the basis of the angiographic appearance at follow-up without evidence of symptoms of myocardial ischaemia.
Angiographic follow-up at six months was available in 36 (90%) patients (three asymptomatic patients refused to return for follow-up catheterisation and one patient died due to lung cancer within a month after the index procedure).
The minimal lumen diameter at baseline, post stent and at follow-up is illustrated in Figure 4. The results of acute and follow-up QCA and IVUS measurements are summarised in Table 3 and Table 4. The binary restenosis rate observed was 12.5% (n=5). The LLL was 0.54±0.3 mm. A total of 15 (37.5%) consecutive patients underwent IVUS at six-month follow-up: Bristol (n=12) and Bonn (n=3). IVUS demonstrated a single case with stent strut malapposition.
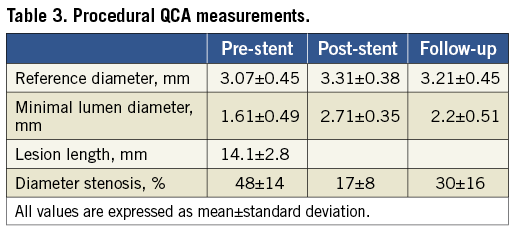
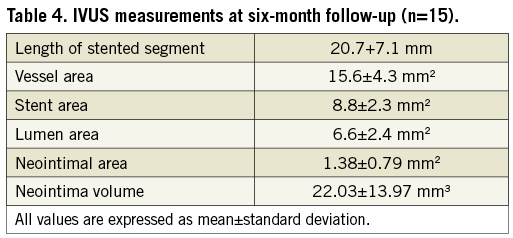
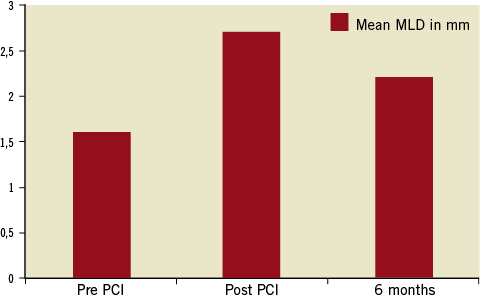
Figure 4. Minimal lumen diameter (MLD) pre PCI, post PCI and at six-month follow-up.
Discussion
This first-in-human experience demonstrated the safety and feasibility of the MOMO cobalt-chromium carbon-coated stent, with excellent acute results, absence of in-hospital major adverse events and a low rate of ischaemia-driven revascularisation (7.5%) and a low LLL at six-month follow-up.
The balloon-expanding MOMO coronary stent is an ultra-thin-strut (70 µm) flexible and conformable stent engineered from a single laser-cut L605 cobalt-chromium hypotube, thus avoiding weld or hinge points. Its closed-cell design provides optimal radial and longitudinal strength (Figure 1). The deliverability of the MOMO stent compares favourably to other commercially available BMS.
In vitro studies have previously shown that stents with DLC coating cause less platelet activation and are less thrombogenic than stainless steel coronary stents15,16,18.
Preclinical evaluation of the MOMO stent in porcine coronary arteries supports the safety and efficacy of this novel stent platform. In a study of three pigs with eight implanted stents, all stented segments remained widely patent with minimal but complete neointimal coverage (<20%). Minimal inflammation was observed around the stent struts with rare giant cells detected and absence of adventitial inflammation (R. Virmani, report on file).
The results of this first-in-human experience confirm the excellent acute performance of the stent platform. The ease of deliverability, flexibility and crossing of the stent across the lesion translated into a 100% technical success. The stent remained stable on the balloon during delivery and positioning in all cases. One case of edge dissection was seen. In this case the stent did not cover the entire length of visible disease and rupture of the mild plaque at the stent edge is a likely event. Sealing of the dissection with a second stent was performed easily.
The long-term results compare well with newer-generation thin-strut bare metal stents9. Ischaemia-driven revascularisation was low (7.5%) at six months and at least as good as other BMS evaluated in similar focal lesion types. In the absence of a protocol-mandated non-invasive ischaemic evaluation, the overall target vessel revascularisation rate (15%) probably reflects in part the result of the well-documented “occulo-stenotic” reflex and may very well have been lower if all patients had received an ischaemia test prior to the mandatory six-month follow-up angiography19. The MOMO six-month LLL of 0.54 mm is lower than that observed with other bare metal stents9,13,20-22, including novel bare metal stents. The titanium-nitride-oxide-coated stent showed an LLL of 0.64 mm at six months23, and the Terumo Tsunami® gold stainless steel stent (Terumo Corp., Tokyo, Japan) had a reported LLL of 0.54 at six months in a comparable series of patients with de novo coronary artery lesions22. These results are better than those seen in older series of bare metal stents. The BENESTENT trial reported an LLL of 0.68 mm and the RAVEL trial BMS arm showed an LLL of 0.8 mm24,25.
Intravascular ultrasound, performed in a subset of patients, confirmed a low neointima volume in the majority of patients. The stents were well expanded, and no significant recoil was noted at six-month follow-up. There was a single case with focal malapposition. This was not linked to an adverse event.
Finally, the absence of stent thrombosis or myocardial infarction with the MOMO stent supports the safety profile of this novel platform.
The selection criteria of lesions shorter than 15 mm and target vessels of at least 3 mm diameter reflect the UK practice, following the guidance of the National Institute for Clinical Excellence (NICE) for selective use of drug-eluting stents26. We did not implant this bare metal stent into lesions and patients who would have been treated with a drug-eluting stent according to our usual practice. Furthermore, we did not enrol patients with multivessel disease, complex bifurcation lesions or left main stem stenosis. Future trials will show acute and long-term results achieved with the MOMO stent in larger and less selective real-world cohorts of patients. A clinical registry is currently under way in The Netherlands.
Study limitations
The present study is designed to examine the safety and clinical feasibility of the MOMO stent in focal lesions within larger vessels. The study does not compare the efficacy of the MOMO stent with other bare metal stents. The study was conducted in three centres with variable operator experience and hence may not have the statistical power to address issues such as MACE during follow-up. IVUS assessment was performed at six months in 15 patients at two centres only.
Conclusion
Preliminary clinical results with this DLC-coated coronary stent are promising. The MOMO stent demonstrates an excellent deliverability and safety profile at six months, and low recurrence rates. Further controlled trials are warranted to validate these preliminary findings.
Acknowledgements
We are grateful to Dr H.P. Hobbach, Siegen, Germany, and Prof Dr H. Omran, Bonn, Germany for their contributions to the study.
Conflict of interest statement
The study was sponsored by Japan Stent Technology, Okayama Research Park Incubation Center, Okayama, Japan. A. Baumbach has received speaker’s fees from Japan Stent for the presentation of results. S. Flohr-Roese received consultancy honoraria from Japan Stent. The other authors have no conflicts of interest to declare.

