
Abstract
Aims: The aim of the study was to investigate the six-month angiographic and nine-month clinical follow-up outcomes in a first-in-man study using the biodegradable polymer-based cobalt-chromium argatroban-eluting stent (JF-04) for treatment of native coronary atherosclerotic lesions.
Methods and results: A total of 31 patients with either stable or unstable angina, or silent myocardial ischaemia, exhibiting de novo coronary lesions were enrolled at seven Japanese sites. The lesions were treated with the JF-04 stent after predilatation. The primary endpoint was angiographic in-stent late loss six months after implantation. The secondary endpoints included angiographic restenosis and in-stent volume obstruction by intravascular ultrasound at six months and target vessel failure (TVF) at nine months. Procedural success was achieved in 100% of cases. At six months, angiographic in-stent late loss was 1.01±0.48 mm and binary restenosis was observed in nine cases (29.0%). Among these restenotic cases, most (n=8) demonstrated advanced angiographic restenosis patterns, including diffuse/proliferative restenosis and total occlusion. At nine months, TVF was observed in four cases (12.9%), exclusively attributed to target vessel revascularisation.
Conclusions: This argatroban-eluting stent failed to inhibit neointimal hyperplasia sufficiently, despite the theoretical benefits and promising clinical experience with local drug delivery.
Introduction
Utilisation of antiproliferative agents delivered locally via drug-eluting stents (DES) has dramatically reduced restenosis rates. On the other hand, the long-term effects of cell cycle inhibitors remain controversial, due to the potential problems of stent thrombosis and late restenosis1. Although current second- or third-generation DES using drugs of the “limus” family significantly alter effectiveness and safety, motivation still exists to pursue alternative approaches using drugs with different mechanisms of action for the further development of DES.
Several phases are thought to be associated with restenotic processes following percutaneous coronary intervention (PCI), including thrombus formation, inflammation, proliferation, and remodelling2,3. Most drugs used in current DES are immunosuppressive agents, and may mainly offset inflammatory and proliferative responses. In contrast, activation of coagulation and thrombus formation have been considered as the initial steps in the mechanism of the arterial restenotic reaction4, which could also be potential targets for the actions of DES.
In several countries, the direct thrombin inhibitor argatroban is frequently used in patients with acute cerebral infarction, and is also used as an alternative agent to heparin in patients with heparin-induced thrombocytopaenia. This agent also inhibits coagulation and thrombus formation within the stent, which may prevent acute/subacute stent thrombosis while also inhibiting neointimal proliferation in the chronic phase. In fact, several animal studies have demonstrated its potential for local delivery in the suppression of vascular restenotic phenomena. Subcutaneous continuous administration of argatroban significantly reduced the luminal stenosis rate following femoral artery injury in mice compared with that of a control solution (unpublished data). Local delivery of this agent successfully suppressed platelet aggregation and smooth muscle cell proliferation after balloon angioplasty in the rabbit carotid artery model5. Furthermore, several human trials with local delivery of this agent demonstrated a significant reduction of the angiographic restenosis rate5,6. Accordingly, the argatroban-eluting stent (JF-04), which is equipped with different characteristics in its actions compared with DES currently on the market, was developed, and was first tested in patients with de novo coronary atherosclerotic lesions. The objective of the first-in-man (FIM) study was to test the safety and feasibility of the JF-04 stent in treating native coronary lesions.
Methods
THE JF-04 STENT
The JF-04 stent (Fukuda Denshi Co., Ltd., Tokyo, Japan) (Figure 1) was loaded with argatroban (C23H36N6O5S·H2O; Mitsubishi Tanabe Pharma Corporation, Osaka, Japan). The stent platform was composed of L605 cobalt-chromium, instrumented with a thin strut thickness of 70 μm and with a closed cell design for more uniform drug delivery. This bare metal stent (BMS) had previously achieved CE mark approval (Angstrom II range of cobalt-chromium stents; Vasmed Technologies, Fujairah, United Arab Emirates). A biodegradable polymer of 50:50 poly (DL-lactide-co-glycolide) was applied as the drug carrier. Two layers of identical biodegradable polymer were splayed, consisting of a basal coating containing argatroban and a top coating without drug. After a dose-finding preclinical study using porcine coronary arteries, a drug dosage of up to 800 μg/cm2 was confirmed to be safe as well as feasible. Considering stent overlaps in actual clinical situations, a drug concentration of 400 μg/cm2 was selected. The in vitro drug-elution pattern displayed in Figure 2 is characterised by: 1) an initial burst release (45% of drug eluted within one day), and 2) completion of elution within 40 days. The basal coating is considered to disappear between six to nine months after implantation. A total of 15 different combinations of stent, comprising three different diameters (2.5, 3.0, and 3.5 mm) and five different lengths (8, 13, 18, 23, and 28 mm) were prepared for this FIM study. An assessment of JF-04 efficacy using the miniature pig artery had demonstrated a similar anti-neointimal proliferation effect as well as lower inflammation scores in comparison with the commercially available first-generation sirolimus-eluting stent (unpublished data).
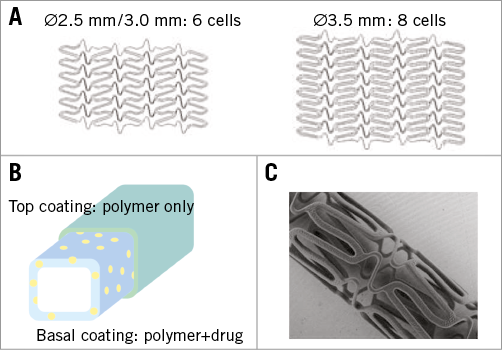
Figure 1. Structural aspects of JF-04. A) Schematic views of stent structure. B) Schematic view of two-layered coating (yellow dots: drug). C) A photograph from scanning electron microscopy (3.5 mm in diameter).
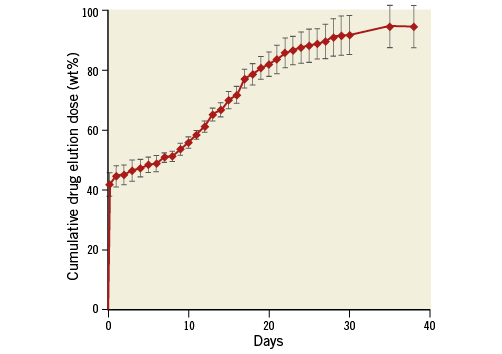
Figure 2. A schema of the drug elution profile of the JF-04 stent, obtained by quantitative measurement of drug dose using ultraviolet-visible spectrophotometry (331 nm) within a single JF-04 stent (3.0×18 mm) bathed in phosphate buffer solution (pH 7.4, 37°C).
STUDY DESIGN AND PATIENT SELECTION
The target lesion was defined as a de novo native coronary artery lesion. A total of 31 patients were enrolled in the study in seven Japanese sites. Patient inclusion criteria included: 1) ≥20 years old, 2) existence of either evident clinical symptoms of stable angina/unstable angina or confirmation of myocardial ischaemia by positive stress tests, 3) single-vessel or double-vessel disease, 4) angiographic lesion length of ≥6 mm, ≤26 mm by visual estimation, and 5) reference vessel diameter ≥2.5 mm, ≤3.5 mm by visual estimation. Exclusion criteria included: 1) left ventricular ejection fraction <30%, 2) existence of acute myocardial infarction (MI) within 72 hours, 3) acute or chronic kidney injury, 4) history of heparin-induced thrombocytopaenia, 4) previous history of BMS implantation into the target vessel within 180 days, 5) previous history of any drug-eluting stent implantation into the target vessel, 6) aortic-ostial location, 7) co-existence of unprotected left main disease, 8) any previous PCI within 30 days, 9) any previous strokes within 90 days, 10) any gastrointestinal bleeding within 180 days, 11) life expectancy less than one year, and 12) existence of side branches ≥2.0 mm in the target lesion.
STUDY PROCEDURE
Lesions were treated by standard interventional procedures under administration of a standard dose of intravenous heparin. Prior to stenting, predilatation by balloon catheter was mandated. Any balloons longer than the stent length could not be used post dilatation. The use of rotational or directional atherectomy, excimer laser, or cutting balloon was prohibited. At least 48 hours prior to the stenting procedure, all patients were given a standard dose of aspirin plus thienopyridine. Dual antiplatelet therapy (DAPT) was mandated for at least 24 weeks after implantation. Subsequent continued lifelong supplementation with low-dose aspirin was recommended, except in those cases where significant side effects were evident.
CORONARY IMAGING
Coronary angiography, intravascular ultrasound (IVUS), and frequency domain optical coherence tomography (FD-OCT) were performed during the procedure and six-month follow-up. Angiograms were obtained from a standard series of multiple projections. All angiographic images were stored on a CD/DVD-ROM for offline analysis. IVUS images were obtained using an Atlantis™ SR Pro 2/OptiCross™ 40 MHz mechanical catheter (Boston Scientific, Marlborough, MA, USA) incorporating an automated pullback system at a speed of 0.5 mm per second. IVUS images were recorded on a CD/DVD-ROM for offline analysis. FD-OCT images were acquired using a Dragonfly™/Dragonfly™ JP/Dragonfly™ OPTIS™ imaging catheter (St. Jude Medical, St. Paul, MN, USA) post procedure and at six-month follow-up. A standardised imaging technique was used with a motorised pullback system at a speed of 18 mm per second. OCT images were digitally stored as raw data and submitted for offline analysis.
IMAGE ANALYSIS
Quantitative coronary angiography (QCA), quantitative coronary intravascular ultrasound (QCU), and OCT image analysis were performed at an independent core laboratory (Cardiocore Japan, Tokyo, Japan). The standard software package, QAngio XA (Medis medical imaging systems bv, Leiden, The Netherlands), was used for QCA. The stented segment and the 5 mm peri-stent segments (proximal and distal to the stent) were analysed by an established standard technique. In each segment, minimal lumen diameter (MLD), reference diameter, and percent diameter stenosis were measured. Late lumen loss was defined as the difference between MLD post procedure and six-month follow-up MLD. Angiographic binary restenosis was defined as %DS ≥50% at six-month follow-up.
A custom software package, echoPlaque™ (INDEC Medical Systems, Santa Clara, CA, USA), was used for QCU assessment. The analysed segment included the stented segment and bilateral 5 mm peri-stent margin segments. The lumen, stent, and external elastic membrane (EEM) boundaries were manually traced at the selected cross-sections. Minimal lumen area (MLA) was selected from the smallest luminal cross-section. Lumen volume, stent volume, and EEM volume were calculated automatically. In-stent neointimal volume was calculated as stent volume minus lumen volume. All volumetric parameters (mm3) were adjusted by length (mm) as “volume index” (mm3/mm) for further data analyses. Qualitative assessment was performed focusing on the presence of incomplete stent apposition (ISA), edge dissection, in-stent thrombus, stent fracture, and plaque protrusion post stenting.
Quantitative FD-OCT analysis was performed using LightLab OCT console software (LightLab Imaging/St. Jude Medical, Westford, MA, USA) every five frames (every 1 mm).
CROSS-SECTION LEVEL ANALYSIS
Lumen area contours were obtained automatically and additional manual corrections were performed if necessary. Stent area contours passing the inner surface of the stent were delineated manually by the point on the centre of the stent reflection7. Intra-stent lumen area contours were delineated at the inner line of either lumen or stent area contour. When the strut was not completely apposed but covered by some material, intra-stent lumen area contours passed the inner edge of covering material. The malapposed area was calculated using the following formula: “lumen area–intra-stent lumen area”. Neointimal (at follow-up phase) areas were calculated using the following formula: “stent area–intra-stent lumen area”. Volumetric measurements were calculated using Simpson’s rule8, and volumetric index was calculated using the following formula: “volume/stent length”.
STRUT-LEVEL ANALYSIS
Distances from strut to lumen area contour (strut-lumen distance) and intra-stent lumen area contour (neointimal thickness) were measured. For accurate and semi-automated measurement, all distances from the struts to contour were automatically parallel to the radial line anchored by the centroid of each contour. Malapposition was adjudicated when the strut-lumen distance was greater than “0.01+0.07 (strut thickness)+0.01 (polymer thickness)” rounded off to two decimal places (in mm). A strut was adjudicated as uncovered when its stent coverage thickness was <0.02 mm because 0.01 mm is recognised as being within the acceptable error range.
CLINICAL ENDPOINT DEFINITIONS
Ischaemia-driven target vessel or lesion revascularisation (TVR, TLR) was conducted if patients met at least one of the following conditions: 1) confirmation of ischaemia by established functional tests, 2) angiographic restenosis (%DS ≥50% by core laboratory measurement) accompanied by evident ischaemic symptoms, and 3) angiographic diameter stenosis ≥70% by core laboratory measurement without positive functional tests or clinical ischaemic symptoms. Myocardial infarction (MI) was defined as a typical rise and gradual fall of troponin accompanied by positive symptoms or new-onset electrocardiogram abnormalities. The ARC standard definition was adopted for judgement of stent thrombosis9. Target vessel failure (TVF) was defined as a composite of target vessel-related cardiac death, MI in the target vessel, and ischaemia-driven TVR.
STUDY ENDPOINTS
The primary endpoint was set as angiographic in-stent late loss at six months after implantation. Secondary endpoints included TVF at nine months and representative angiographic, IVUS, and OCT parameters, including binary restenosis rate, MLD, neointimal volume, neointimal thickness, strut coverage rate, and frequency of stent fracture, ISA, and in-stent thrombus.
STATISTICAL ANALYSIS
All analyses were performed according to the intention-to-treat principle. Continuous variables are presented as means±standard deviation, or median and interquartile range (IQR) if these were not normally distributed. Categorical variables are presented as counts and percentages. Paired t-tests or Wilcoxon matched pairs signed-rank tests were performed between post procedure and six-month follow-up. A p-value <0.05 was considered as statistically significant. All statistical analysis was performed with SAS, Version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
PATIENT, LESION, AND PROCEDURAL CHARACTERISTICS
A total of 31 patients were enrolled in this study between February and September 2013. Baseline clinical, lesion, and procedural characteristics are summarised in Table 1. Device success was achieved in 100% of cases. Most patients were treated with a single study stent (87.1%). Two cases discontinued DAPT within the initial 24 weeks: one case quitted both antiplatelet agents due to scheduled urological surgery and one case discontinued aspirin for 14 days on the referral doctor’s judgement. Twenty-six patients (83.9%) continued DAPT at the time of clinical endpoints (nine-month follow-up).
ANGIOGRAPHIC OUTCOMES
Table 2 demonstrates serial quantitative and qualitative coronary angiography at baseline and follow-up. MLD significantly decreased from post intervention to follow-up. In-stent late loss, the primary endpoint of this study, was 1.01±0.48 mm. In-segment late loss was 0.74±0.51 mm. The angiographic binary restenosis rate was 29.0%. Figure 3 shows cumulative frequency distribution curves for in-segment MLD at pre-intervention, and in-stent MLD at post intervention and six-month follow-up. In cases of restenosis, advanced angiographic patterns, such as diffuse, proliferative, and silent occlusion, predominated (eight of nine restenotic cases).
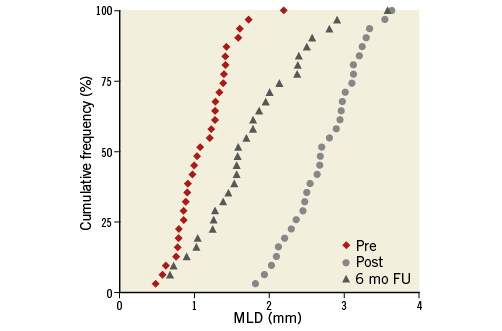
Figure 3. Cumulative frequency distribution of in-segment minimal lumen diameter at pre-intervention, and in-stent minimal lumen diameter at post intervention and six-month follow-up.
INTRAVASCULAR ULTRASOUND ANALYSIS
IVUS evaluation at six-month follow-up was performed in 30 patients (96.8%). In one case this was not possible, due to the presence of totally occlusive restenosis. Serial quantitative and qualitative assessments are shown in Table 3. A significant lumen volume reduction was observed with an in-stent volume obstruction of 31.9±13.7%. ISA was seen in 12 patients (38.7%) at baseline, whereas this finding was resolved in 11 patients and persistent in only one patient at follow-up. No evident thrombus, dissection, or stent fracture was documented at either post procedure or follow-up.
FD-OCT ANALYSIS
FD-OCT evaluation at six-month follow-up was also performed in 30 of 31 patients (96.8%) for the same reason as indicated for IVUS assessment. In one case, whole segment analyses could not be performed due to insufficient image quality, because of incomplete washout of red blood cells. The results of serial quantitative and qualitative FD-OCT assessments are shown in Table 4. Mean neointimal thickness was 0.38±0.14 mm. Although malapposed struts were documented in nearly 3% of struts at baseline, this finding had almost disappeared at follow-up. Uncovered struts were quite rare at six-month follow-up.
CLINICAL OUTCOMES
Table 5 shows adverse cardiac events at nine-month follow-up. No stent thrombosis occurred during the nine-month period. TVF was 12.9%, which was exclusively attributed to ischaemia-driven TVR.
Discussion
This FIM experience successfully established excellent results in the acute phase and also the safety of the JF-04 stent. On the other hand, it failed to demonstrate sufficient suppression of in-stent neointimal hyperplasia, despite the fact that this study was not powered to test efficacy. In fact, the angiographic in-stent late loss of 1.01±0.48 mm was considered large in comparison with that of approved DES. It appears to be similar to or even larger than that of other BMS. Similarly, an in-stent volume obstruction of 31.9±13.7% was also relatively large for a DES. While the results obtained were rather disappointing, the potential factors or mechanisms responsible for such outcomes should be rigorously assessed to provide lessons and reference points for future DES development.
First of all, the theoretical relevance of using antithrombin agents for inhibition of restenosis is now discussed. In general, vessel injury and mechanical irritation provoked by stent implantation initially lead to a burst in activation and adhesion of thrombocytes, generating the thrombus at the site of vessel denudation10. The stent, which is a metallic foreign body, enhances thrombus formation by activating the blood coagulation cascade and the adhesion of fibrinogen to the stent surface11. Subsequently, intense infiltration of monocytes and cells of the immune system occurs into the thrombocyte-fibrin layers. Adhesion of leukocytes is thought to be the secondary inducer of neointimal proliferation after platelet aggregation at the injury site10, producing large quantities of proliferation-stimulating substances. A series of these responses occurs within the first few days. Therefore, “control” of thrombus and fibrin formation at very early stages could theoretically become the target for inhibition of stent thrombosis and restenosis.
Historically, heparin has been widely evaluated for such a purpose. Several trials of a heparin-coated stent showed the potential for reduction of stent thrombosis and/or suppression of neointimal proliferation12. However, a randomised trial comparing heparin-coated stents and identically designed uncoated stents failed to show positive influences on restenosis or clinical outcome13. Although some potential remained to be explored using this technology, sirolimus- and paclitaxel-eluting stents were developed in parallel at that time14,15. Once the potential performances of the latter were recognised, interests and efforts were completely diverted to development of these potent DES equipped with antiproliferative agents such as the limus family or antineoplastic agents.
However, several safety concerns related to first-generation stents started to emerge and immediately became the most urgent issues to be resolved16. These facts rekindled the motivation for development of optional DES exhibiting other strengths, such as the capacity to promote early vascular healing or enhanced maximised safety. Thus, a DES equipped with the antithrombin agent argatroban was developed and tested in this study. However, the present FIM study failed to demonstrate its efficacy.
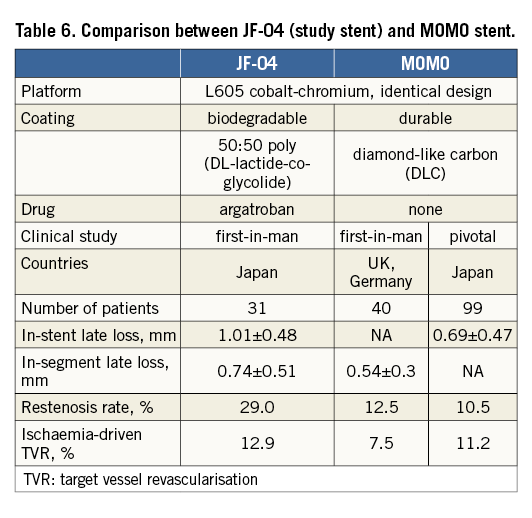
The MOMO stent (Japan Stent Technology Co., Ltd., Okayama, Japan) is structurally related to the JF-04 stent, incorporating an identical platform, though a different type of coating in the absence of drug. It has already been evaluated in several studies17, which successfully demonstrated relatively smaller in-stent late loss compared with that of representative BMS. Table 6 displays a comparison between these two stents. Surprisingly, in-stent late loss with the argatroban-eluting stent appeared to be greater than that of the non-drug-eluting stent. Detailed assessments and appropriate interpretation need to be undertaken to understand such unexpected outcomes. One can speculate on several possibilities. First, initial burst drug release could be problematic. Clearly nearly half of the drug (45%) is released in the early phase, which may have repercussions regarding efficacy. Second, argatroban alone may be insufficiently potent to control “in-stent” neointimal tissue proliferation. Although it acts via local drug delivery after balloon angioplasty to some extent, a stronger antiproliferative performance may be required in the presence of additional extraneous material such as the coronary stent. Similarly to previous clinical studies of other DES using different rationales of drug action, i.e., heparin13, oestrogen18, and tacrolimus19, such indirect or mildly antirestenotic agents could not achieve acceptable outcomes in situ. Furthermore, the hypothesis regarding the action of the argatroban-eluting stent, namely that “inhibition of the initial steps of the restenotic phenomenon by suppression of thrombosis and fibrin formation may also prevent neointimal hyperplasia in the chronic phase”, may not be realistic in the first place.
Third, a “gap” in the time course of absorption between drug and polymer may be the problem. Argatroban is considered to be completely released within 40 days, yet polymer needs more than half a year to be fully absorbed. We speculate that such a “gap” may underlie the mechanisms of the occurrence of neointimal overgrowth compared with the MOMO stent; the latter demonstrated a worse outcome, even with adoption of a potentially effective agent onto the strut surface. A previous animal study also suggested that the balance between drug levels and the coating products is important for sufficient neointimal suppression by DES20. In fact, several newest-generation DES equipped with a minimised gap in duration of absorption (within around one month) have shown excellent clinical outcomes21. Some pharmacological supports may be required to act against vascular responses due to polymer absorption.
From this FIM study, we speculate that 1) the antiproliferative capability of the drug, 2) the phase in the restenotic process on which it acts, 3) drug elution speed, and 4) the temporal gap between drug and polymer absorption, may be critically important for biodegradable polymer-based DES. These discussions will become valuable lessons for consideration in the future development of DES actions.
Conclusion
The first-in-man study of a biodegradable polymer-based argatroban-eluting stent demonstrated safety, but failed to show sufficient inhibition of neointimal hyperplasia in de novo native coronary lesions, despite theoretical benefits and promising clinical experience with local drug delivery.
| Impact on daily practice The JF-04 stent, equipped with the direct antithrombin agent argatroban coupled with a biodegradable polymer, was developed and tested in 31 patients, after successful animal and clinical investigations of its local delivery following balloon angioplasty. Despite the theoretical benefits and promising former experiences, this first-in-man study failed to demonstrate its efficacy. Future specifications for biodegradable polymer-based DES may include the antiproliferative capability of the drug, the phase in the restenotic process on which it acts, drug elution speed, and the temporal gap between drug and polymer absorption, which must provide valuable lessons for consideration in the future development of drug-eluting stent (DES) actions. |
Acknowledgements
We appreciate the efforts of all members of the participating centres.
Funding
The study was sponsored by Fukuda Denshi Co., Ltd.
Conflict of interest statement
Y. Morino has received a research grant and speaker’s fee from Fukuda Denshi. K. Kataoka has received a consulting fee and speaker’s fee from Fukuda Denshi. The other authors have no conflicts of interest to declare.

