Abstract
Aims: This study sought to evaluate the safety and efficacy of the NOYA stent which is a cobalt chromium-based sirolimus-eluting stent (SES) with DL-polylactide biodegradable polymer (Medfavour Medical, Beijing, China) in treating de novo coronary artery lesions.
Methods and results: The NOYA I trial was designed to compare the NOYA stent with the FIREBIRD2™ stent, a durable polymer SES widely used in China (MicroPort Medical, Shanghai, China); the trial was a non-inferiority trial with a primary angiographic endpoint of the in-stent late lumen loss (LLL) at nine-month follow-up. The secondary endpoints were binary restenosis rates within nine months, major adverse cardiac events (MACE) defined as the composite of cardiac death, myocardial infarction (MI) or target lesion revascularisation (TLR), and definite/probable stent thrombosis (ST) at 24-month follow-up. A total of 300 patients (n=150 in each group) were enrolled in the study from 16 Chinese centres. The LLL in the NOYA group at nine-month follow-up was similar to the FIREBIRD2 group (0.11±0.18 mm vs. 0.14±0.23 mm, p=0.16; non-inferiority p<0.001). The rates of MACE, death, MI and TLR at 24-month follow-up were comparable between these two devices (p>0.05, respectively).
Conclusions: The biodegradable polymer NOYA stent was non-inferior to the FIREBIRD2 durable polymer stent with respect to the primary non-inferiority endpoint of in-stent LLL at nine-month follow-up. Clinical outcomes at 24-month follow-up were comparable between the two stents. (ClinicalTrials.gov number, NCT01226355)
Introduction
As compared to bare metal stents (BMS), drug-eluting stents (DES) have been proven as a standard mechanical device for percutaneous coronary intervention (PCI).1-4 The introduction of DES has greatly improved the percutaneous management of coronary artery lesions as it has reduced the risk of restenosis, especially in complex cases and high-risk patients. However, concerns have been raised with respect to a potential increased risk of stent thrombosis (ST) in the treatment of patients with coronary artery disease (CAD) after DES implantation.5,6 It has been noted that durable polymer carriers may play a significant contributory role in DES-related hypersensitivity reactions and delayed vessel healing, which may subsequently contribute to the frequency of late and very late ST.7-9 To address this issue, a newer generation of DES has been developed which uses biodegradable polymer carriers in order to decrease these long-term complications. After full degradation of these polymers into carbon dioxide and water, once complete drug release has been ensured, the polymers leave behind a residual bare metallic stent.10,11
The present study sought to evaluate the safety and efficacy of the NOYA biodegradable polymer-based, sirolimus-eluting stent (SES) (Medfavour Medical, Beijing, China), in the treatment of patients with de novo coronary artery lesions.
Methods
STUDY DESIGN AND PATIENT POPULATION
A prospective, single-blinded, multicentre, randomised clinical trial was designed to demonstrate the non-inferiority of the NOYA biodegradable polymer-based SES compared to the FIREBIRD2 SES (MicroPort Medical, Shanghai, China), which is widely used in China. A total of 300 patients were enrolled in the present study at 16 centres in China from April 2009 to November 2009. (ClinicalTrials.gov Identifier: NCT01226355)
The study protocol was approved by the respective hospital ethics committees and monitored by the Division of Biometrics, Centre for Cardiovascular Disease Control and Prevention, Ministry of Health, China. Written informed consent was obtained from all patients.
The inclusion criteria were as follows: age 18-75 yrs old, maximum two de novo native coronary artery lesions; reference vessel diameter (RVD) 2.25-4.0 mm, lesion length ≤30 mm at target lesion; patient willing to receive clinical and angiographic follow-up. The exclusion criteria included acute myocardial infarction (AMI) within one week; left main coronary artery lesion; total occlusion lesion (CTO, with TIMI grade 0 flow); true bifurcation lesion with side branch diameter >2.25 mm; NYHA classification >Class III or LVEF <40%; restenotic lesion; prior PCI within 12 months.
STUDY DEVICES
The NOYA stent (Figure 1) is a newly developed sirolimus-eluting coronary stent with a unique open-cell design and a thin (81 microns) cobalt-chromium platform, coated with a DL-polylactide biodegradable polymer containing the antiproliferative drug sirolimus. Kinetic release data (Figure 2) indicate that about 87% of the drug releases in one month and full absorption of the coating takes place over four months. The available stent lengths are 8, 13, 18, 23, 28, 33, and 38 mm with diameters of 2.25, 2.5, 2.75, 3.0, 3.5, and 4.0 mm.
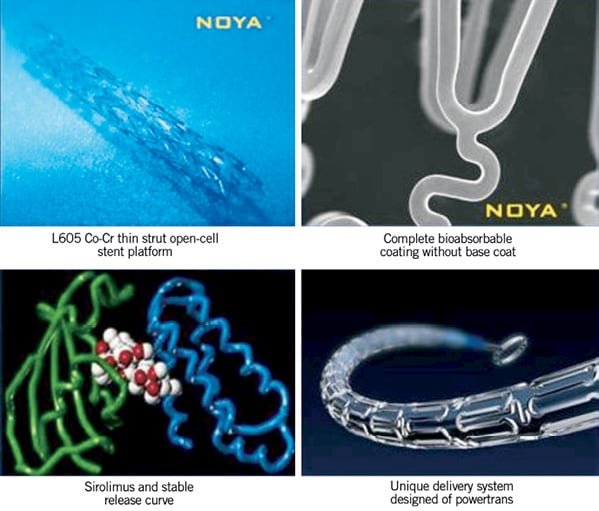
Figure 1. Components of the NOYA biodegradable polymer-based sirolimus-eluting stent.
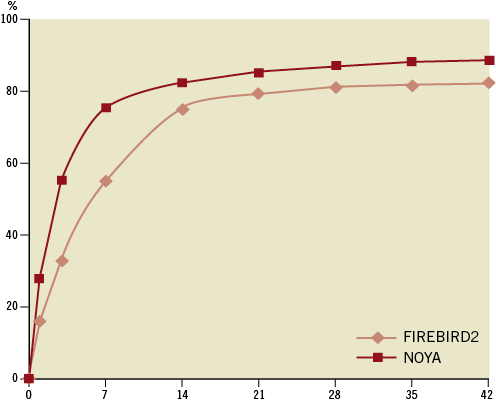
Figure 2. Comparison of the drug elution curve.
PROCEDURAL AND POST-INTERVENTIONAL PRACTICES
The interventional strategy was determined at the discretion of the individual cardiologist. Administration of 300 mg clopidogrel and 300 mg aspirin as loading doses within the 24 hrs before the procedure was mandatory. At least 12 months of both clopidogrel (75 mg/day) and aspirin (100 mg/day) were prescribed to all patients.
FOLLOW-UP AND THE STUDY ENDPOINTS
Clinical follow-up was scheduled at 30, 90, 180, 270, 360, and 720 days after the initial procedure. Angiographic follow-up was obtained at baseline and at 270 days. The primary endpoint was 270-day late lumen loss (LLL). The secondary endpoint included in-stent and in-segment binary restenosis at the 270-day angiographic follow-up, MACE (the composite of death, myocardial infarction [MI], or target lesion revascularisation [TLR]), and ST during the clinical follow-up period.
Death was divided into cardiac death and non-cardiac death. Any death due to proximate cardiac cause (e.g., MI, low-output failure, fatal arrhythmia), unwitnessed death and death of unknown cause, and all procedure-related deaths, including those related to concomitant treatment, were classified as cardiac death. MI was defined as elevation of creatine kinase CK-MB >3 times the upper limit of normal with or without new pathological Q-waves on the electrocardiogram. Procedural success was defined as achieving <20% residual stenosis with TIMI grade 3 flow of all lesions attempted without major clinical complications. ST was defined according to Academic Research Consortium (ARC) definitions.12 All events were adjudicated by an independent clinical events committee.
Coronary angiograms were obtained in multiple views after an intracoronary injection of nitroglycerine (200 micrograms) at pre- procedure, post procedure and 270-day follow-up. Quantitative coronary angiography (QCA) analysis was performed by an independent core laboratory (CCRF, Beijing, China) using QAngio® XA system (Medis Medical Imaging Systems, Leiden, The Netherlands). In each patient, QCA measures within the stent and the analysis segment (including the stented region and 5 mm edge regions) were analysed and reported separately. The LLL was defined as the difference between post-procedural and 270-day follow-up minimal lumen diameter (MLD). Binary restenosis was defined as ≥50% diameter stenosis at follow-up, and was classified as in-stent if inside the stent or in-segment if located either within the stent segment or up to 5 mm proximal or distal to the stent.
STATISTICAL ANALYSIS
The primary objective was to demonstrate the non-inferiority of the NOYA stent compared to the FIREBIRD2 stent with respect to angiographic in-stent LLL. The estimated in-stent LLL of the control group was 0.14±0.24 mm, which was derived from unpublished data in the FIREBIRD2 FIC (Firebird-in-China) registry study. We assumed the NOYA group would have the degree of LLL with a non-inferiority margin of 0.12 mm. Using a two-sided test with an alpha level of 0.05 and assuming a 40% loss to angiographic follow-up, we calculated that 300 patients would yield more than 90% power to detect non-inferiority. Continuous variables were expressed as mean ± standard deviation and compared through the use of the Student’s t-test. Categorical variables were expressed as percentages and the chi-square test and Fisher’s exact test were used for comparisons. An ANOVA analysis was used as the primary analysis for LLL. The prespecified covariates in this model were the post-procedure MLD and the investigation site. Cumulative incidence of MACE at two years was estimated by the Kaplan-Meier method and compared by use of the log-rank test. All statistical tests were two-sided, and a p<0.05 was considered significant. All analyses were performed with the SAS 9.13 system (SAS Institute, Cary, NC, USA).
Results
BASELINE AND PROCEDURAL CHARACTERISTICS
Baseline patient characteristics were comparable between the two study groups (diabetes, 22% vs. 20%; prior PCI, 8% vs. 9.3%; unstable angina, 39.2% vs. 51.1%, in the NOYA and in the FIREBIRD2 groups respectively) (Table 1).
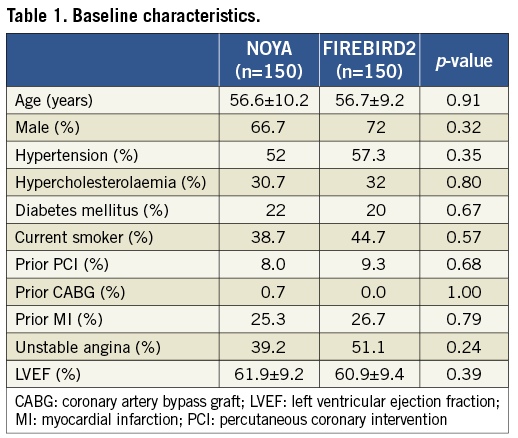
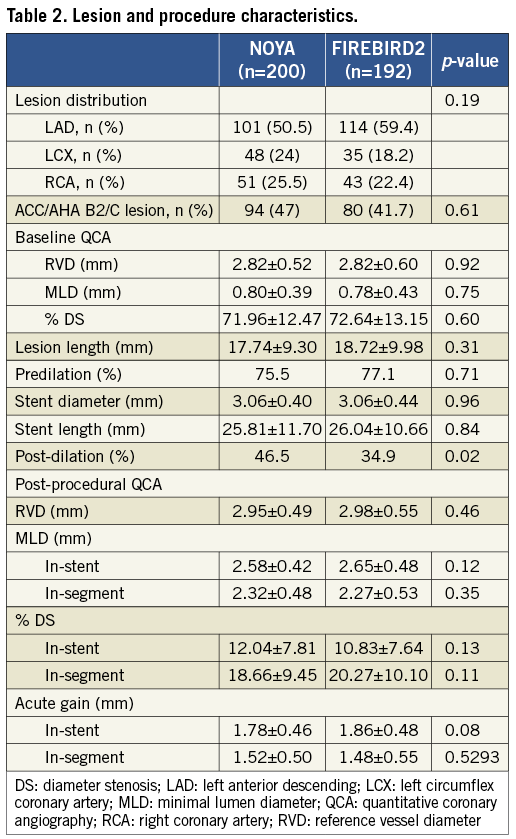
Although a greater frequency of post-dilatation was found in the FIREBIRD2 group, other lesion and procedure characteristics were comparable (B2-C lesion, 47% vs. 41.7%; stent diameter, 3.06±0.40 mm vs. 3.06±0.44 mm; stent length, 25.81±11.70 mm vs. 26.04±10.66 mm, in the NOYA and FIREBIRD2 groups respectively) (Table 2). The rate of device success was 100% in both groups.
ANGIOGRAPHIC FOLLOW-UP
The percentage of patients who underwent angiographic follow-up at 270 days was 84.7% after the initial procedure (NOYA: 127/150, with 167 lesions; FIREBIRD2: 127/150, with 164 lesions) (Table 3). Angiographic LLL at nine months in the NOYA group was similar to the FIREBIRD2 group (0.11±0.18 mm vs. 0.14±0.23 mm, p=0.16; absolute difference [95% confidence interval]: -0.03 [-0.08, 0.01]; non-inferiority: p<0.001). The rates of in-stent binary restenosis (NOYA 1.8% vs. FIREBIRD2 0.6%, p=0.62) and in-segment binary restenosis (NOYA 4.2% vs. FIREBIRD2 3.7%, p=0.80) were similar in both groups. The cumulative distribution curves of MLD pre-procedure, post procedure and at angiographic follow-up between the two groups are shown in Figure 3 and LLL in Figure 4. As a surrogate indicator of late malapposition to some extent, the incidence of the negative LLL was comparable between the two populations treated either by the NOYA or by the FIREBIRD2 stent (18.9% vs.14.4%, p=0.297).
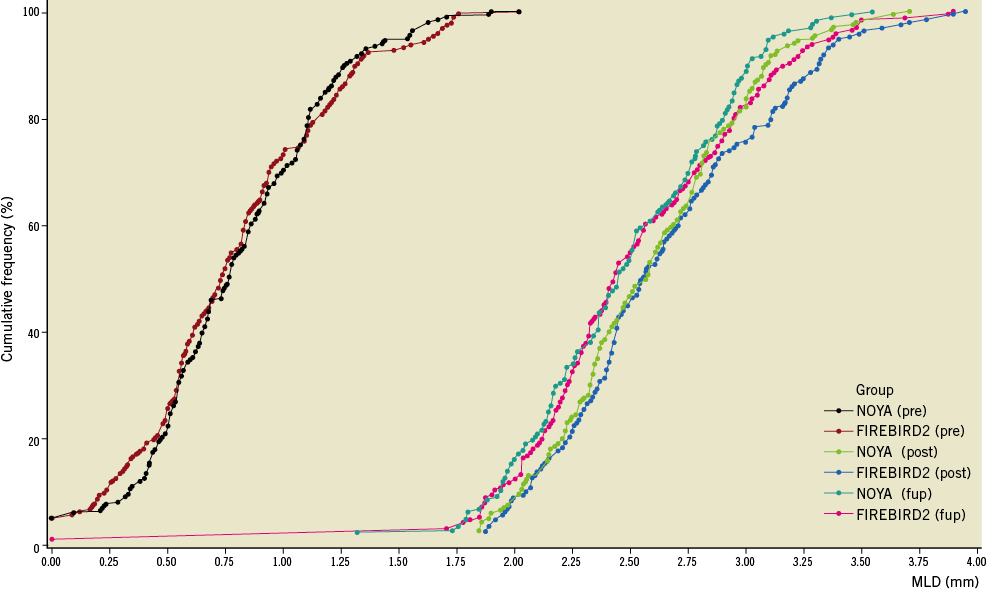
Figure 3. Cumulative distribution curves of MLD pre-procedure, post-procedure and at angiographic follow-up.
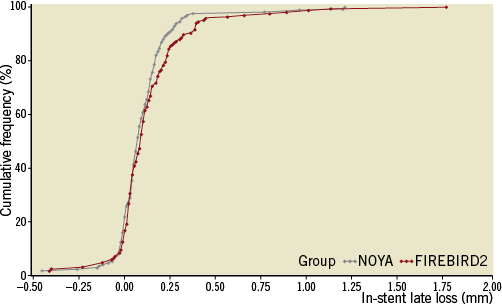
Figure 4. Cumulative distribution curves of the LLL.
CLINICAL OUTCOMES
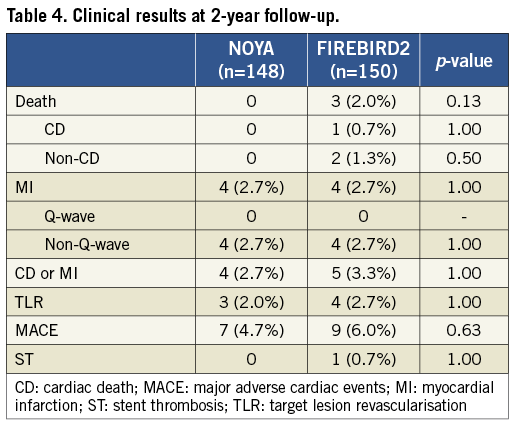
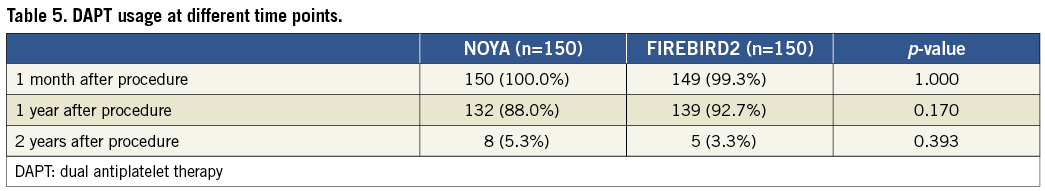
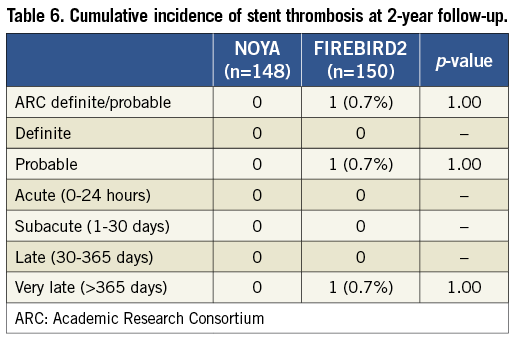
Clinical data were available for 99.3% of the patients in the current study at two-year follow-up (Table 4). Similar clinical outcomes were observed between the NOYA and FIREBIRD2 groups at 24-month follow-up (MACE 4.7% vs. 6.0%; death 0 vs. 2.0%; MI 2.7% vs. 2.7%; TLR 2.0% vs. 2.7%, respectively). The percentage of patients who received dual antiplatelet therapy treatment was similar between the NOYA group and the FIREBIRD group at one-month, one-year and two-year follow-up (Table 5). Only one case of stent thrombosis was observed in the present study (Table 6).
Discussion
The presence of permanent polymer coatings on DES may have pro-inflammatory and potentially thrombogenic effects. Hence, much current DES research has focused upon studies of biodegradable polymer coatings or polymer-free DES platforms. The present study is an early randomised clinical trial studying the use of biodegradable polymer-based, sirolimus-eluting stents in treating patients with CAD in China. The study demonstrated that the NOYA biodegradable polymer SES had similar safety and efficacy compared to the FIREBIRD2 durable polymer SES.
In the present study, due to its widespread use in China and its previous safety and efficacy record after implantation in patients, the FIREBIRD2 SES was chosen as a control13-16. The effective antiproliferative effects of sirolimus on the vessel at the location of the target lesion are likely to be a crucial determinant of favourable clinical outcomes with SES17,18. Despite differences in the polymer carrier, similar angiographic and clinical efficacy was observed between the NOYA stent and the FIREBIRD2 stent. The similar doses of sirolimus loaded on either stent (NOYA 8.8 μg/mm, FIREBIRD2 9 μg/mm) and the similar drug elution characteristics (Figure 2) may have accounted for the consistent clinical outcomes in the two groups.
This trial was not adequately powered to address safety endpoints. It is nonetheless reassuring that the incidences of death, MI and thrombosis were all low and comparable between the two groups in the present study. Due to the limited study population, in addition to longer-term safety concerns with DES, in order to evaluate fully the safety of NOYA stent implantation, much larger patient groups with longer clinical follow-up will be necessary.
In order to diminish the pro-inflammatory and potentially thrombogenic potential of the permanent polymer coatings of existing DES, polymer-free DES platforms have been designed. However, in a recently published trial, a DES platform without a polymer coating, designed to elute paclitaxel or sirolimus, failed to show non-inferiority compared with the first-generation DES with durable polymer coating19,20. Mehilli et al20 also demonstrated that a polymer-free stent eluting sirolimus provided an inferior efficacy, but a biodegradable polymer stent eluting sirolimus was at least as effective as the permanent polymer SES in terms of antirestenotic efficacy. Hence, current polymer-free stent platforms for drug release may have a less bright future, but biodegradable polymer stents may provide both effective antiproliferation and may remove inflammation caused by the polymer. With improvements in technology, bioabsorbable scaffolds providing transient vessel support with drug delivery capability, without the long-term limitations of drug-eluting stents, have also achieved some success for the treatment of coronary artery disease21,22. However, although biodegradable stents cannot be used for complicated coronary lesions, biodegradable polymer stents will still play an important role in the field of interventional cardiology for the treatment of coronary artery disease.
Study limitations
The limitations of the study are as follows: 1) patients included in the study were selected strictly by angiographic and clinical characteristics; 2) in the present study, the absence of intracoronary imaging examinations (such as IVUS, OCT) was limited to evaluate the additional measurements for the new stent; 3) the trial was a modestly sized randomised trial with angiographic endpoints (and angiographic follow-up). In the future, a large-scale randomised trial conducted on less stringently selected patients will be needed to determine the long-term efficacy and safety of this novel sirolimus-eluting coronary stent with bioabsorbable coating.
Conclusions
Based on the present study, the NOYA biodegradable polymer-based SES appears to be a safe, effective and feasible treatment option for patients with de novo coronary artery disease. Large-scale trials with long-term follow-up are needed to evaluate further this newly developed SES with biodegradable polymer coating.
Acknowledgements
We are grateful not only to all of the investigators of the NOYA I trial for their hard work but also to Medfavour Medical (Beijing, China) for its support.
Conflict of interest statement
Y. Yang, S. Lu, L. Wang, H. Wang, Z. Li, L. Wang, Y. Chen, W. Li, and R. Gao received research grants from Medfavour Medical. All the other authors have no conflicts of interest to declare.

