
Abstract
Aims: Long-term data on bioabsorbable polymer-coated everolimus-eluting stents (BP-EES) are limited. The EVOLVE trial compared the safety and efficacy of two dose formulations of the SYNERGY BP-EES with the permanent polymer-coated PROMUS Element EES (PE).
Methods and results: The EVOLVE study was a prospective, multicentre, non-inferiority trial that randomised 291 patients with de novo coronary lesions (length: ≤28 mm; diameter: ≥2.25 to ≤3.5 mm) to receive PE (n=98), SYNERGY (n=94), or SYNERGY half-dose (n=99). At five years, there were no significant differences in the rates of TLF or individual components between groups. TLR rates trended lower in both SYNERGY arms than in the PE arm (TLR: 1.1% SYNERGY and 1.0% SYNERGY half-dose vs. 6.1% PE; p=0.07 and p=0.06, respectively). TVR was numerically lower in the SYNERGY arms compared to the PE arm (TVR: 3.3% SYNERGY and 4.2% SYNERGY half-dose vs. 10.2% PE; p=0.06 and p=0.11, respectively). No incidence of stent thrombosis was reported in any arm up to five years.
Conclusions: The EVOLVE trial represents the longest-term follow-up of the SYNERGY stent available to date, demonstrating its continued safety and efficacy for the treatment of selected de novo atherosclerotic lesions up to five years.
Abbreviations
BP: bioabsorbable polymer
DAPT: dual antiplatelet therapy
MI: myocardial infarction
PE: PROMUS Element
PP-DES: permanent polymer-coated drug-eluting stent
ST: stent thrombosis
TLF: target lesion failure
TVR: target vessel revascularisation
Introduction
Despite significant reduction in restenosis compared to bare metal stents, permanent polymer-coated drug-eluting stents (PP-DES) can delay healing of the arterial wall and increase inflammation, which may be associated with late/very late stent thrombosis (ST)1. DES with bioabsorbable polymers (BP) were designed to mitigate these effects, thereby potentially reducing late events and the need for prolonged dual antiplatelet therapy (DAPT)2,3. Some studies have suggested improved safety with BP-DES compared to PP-DES4; however, long-term data are limited.
SYNERGY™ (Boston Scientific, Marlborough, MA, USA), a thin-strut platinum-chromium, everolimus-eluting stent abluminally coated with an ultrathin BP, was non-inferior for six-month in-stent late loss (at two dose formulations) compared with the PP-coated PROMUS Element™ EES (PE; Boston Scientific) in the EVOLVE first human use trial2,5. Major cardiac endpoints up to two years were not significantly different between groups2,5. Herein, we describe the final five-year outcomes of the EVOLVE trial.
Methods
The EVOLVE trial randomised 291 patients with de novo coronary lesions (length: ≤28 mm; diameter: ≥2.25 to ≤3.5 mm) to receive PE, SYNERGY, or SYNERGY half-dose. Clinical study design and primary endpoints have been described previously2,5. Secondary endpoints included death, myocardial infarction (MI), target vessel revascularisation (TVR) and ST (ARC definitions). Events up to five years were adjudicated by an independent clinical events committee blinded to treatment assignment.
Results
Baseline clinical, lesion and procedural characteristics, described previously2, were generally similar between groups. Five-year clinical follow-up was completed in 99.0%, 95.7% and 96.0% of PE, SYNERGY and SYNERGY half-dose patients, respectively. At five years, DAPT usage was low – 16.7% (PE), 12.2% (SYNERGY) and 14.8% (SYNERGY half-dose).
Kaplan-Meier curves for target lesion failure (TLF) between one and five years were flat and parallel between arms, showing accrual of very few events (Figure 1A). TLF and individual components at five years were not significantly different between the SYNERGY and PE arms (Figure 1B). The five-year TLR rate trended lower in both SYNERGY groups compared to PE. No subject in any treatment arm experienced a definite/probable ST up to five years (Table 1).
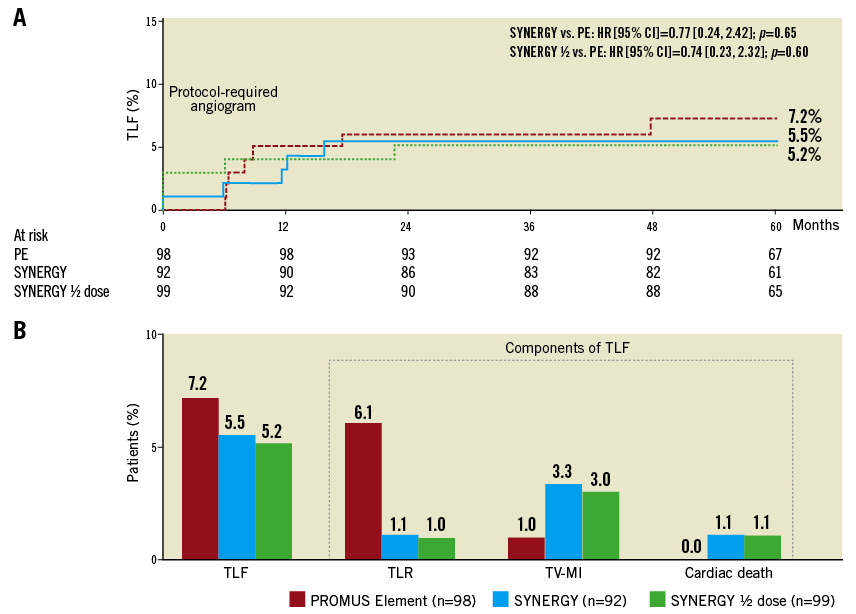
Figure 1. TLF and components at five years. A) Cumulative Kaplan-Meier curve for TLF up to five years. B) Time-to-event rates for TLF and components at five years. A six-month angiogram was required, per protocol. HR: hazard ratio
Discussion
Data from the EVOLVE trial represent the longest follow-up available with SYNERGY. At five years, no significant differences in clinical event rates between patients implanted with SYNERGY, SYNERGY half-dose or PE were found. No ST was reported, and cardiac mortality was similar between arms. Overall mortality was higher with SYNERGY, driven by deaths due to cancer, trauma and infection, probably reflecting the play of chance. Although not powered to detect differences in clinical outcomes due to sample size, data presented here support the long-term safety and efficacy of SYNERGY in selected patients/lesions2,5.
Long-term follow-up of trials evaluating BP-DES are limited. Compared to other trials with PP-EES6,7, TLR up to five years was at least comparable, if not lower. EVOLVE was a non-inferiority study, not designed to assess potential benefits of BP-EES vs. PP-EES. The EVOLVE II randomised non-inferiority trial3 and EVOLVE II Diabetes Substudy8 demonstrated the safety and efficacy of SYNERGY in a larger and broader patient population, consistent with the favourable long-term outcomes observed in this study. Data from numerous ongoing trials evaluating SYNERGY in more complex patient populations (EVOLVE Short DAPT, POEM, MULTISTARS AMI, SYNTAX II and IDEAL LM) will provide additional insight into the benefits of BP-EES.
Limitations
The results presented here may not apply to complex patients/lesions; however, SYNERGY was non-inferior to PE in the all-comers patient population in the EVOLVE II trial3.
Conclusions
Five-year data from the EVOLVE non-inferiority trial demonstrated low and comparable clinical event rates between SYNERGY, SYNERGY half-dose and PE-treated patients. This is the longest-term study with SYNERGY available to date and, therefore, provides valuable information on its safety and efficacy in patients with simple lesions.
| Impact on daily practice The EVOLVE trial demonstrated low rates of TLR, TLF and cardiac death in both BP-EES and PP-EES at five years; no ST was reported in any treatment arm. These outcomes confirm and expand the data previously reported for SYNERGY in the treatment of selected de novo coronary lesions. |
Acknowledgements
We wish to thank Pooja Bhatt, PhD, and Songtao Jiang, MSc, (both BSC) for assistance in manuscript preparation and statistical analysis.
Funding
EVOLVE was supported by BSC.
Conflict of interest statement
I. Meredith (from January 2017, after completion of EVOLVE), T. Christen, and D. Allocco are full-time employees of and have equity interest in Boston Scientific Corporation (BSC). C. Dubois is on the scientific advisory board of and has received honoraria from BSC. B. Farah and D. Carrié have received honoraria from BSC. J. Dens and S. Walsh have received research funding and consulting fees from BSC. K. Oldroyd has received honoraria and research support from BSC. O. Varenne has received research support from BSC. S. El-Jack reports receiving research support from BSC, Abbott and Medtronic, and is on the advisory board for Medtronic. R. Moreno reports receiving honoraria from BSC, Abbott Vascular, St. Jude Medical, Medtronic, Biotronik, Daiichi Sankyo, Amgen, Astra, and Biosensors. The other author has no conflicts of interest to declare.

