Abstract
Aims: Previous studies for approved indications (on-label) have shown the good safety and efficacy profiles of the Nobori DES. We conducted a prospective, multicentre study to validate the clinical performance of this stent in a real-world setting.
Methods and results: A total of 3,067 consecutive patients undergoing a percutaneous coronary intervention with the Nobori DES were enrolled in the NOBORI 2 registry. At one and two years, 97% and 95% of patients, respectively, were available for follow-up. The rates of target lesion failure (TLF), cardiac death, myocardial infarction and target lesion revascularisations were: 3.9%, 1.2%, 1.9% and 2.2% at one year and 5.1%, 1.6%, 2.4% and 3.0% at two years. Overall, 2,242 patients (73%) were treated for at least one off-label indication. When comparing off-label and on-label groups, the results were: TLF 4.5% vs. 2.2%, p=0.003 at one year and 5.9% vs. 2.8%, p=0.001 at two years. The rate of stent thrombosis was 0.68%, and 0.80% at one and two years, respectively with no difference between the off-label and on-label groups (0.76% vs. 0.48%, p=0.6 and 0.89% vs. 0.61%, p=0.5).
Conclusions: The promising results previously observed in lower risk patients can be replicated in daily practice. As expected, in off-label indications, rates of adverse events were higher. Nevertheless, our results suggest the good and sustained performance of this stent system in high-risk patients with significant comorbidities and/or complex lesions. (Clinical trial registration: ISRCTN81649913; http://www.controlled-trials.com/isrctn/search.html?srch=81649913&sort=3&dir=desc&max=10)
Introduction
Drug-eluting stents (DES) are widely used for patients undergoing percutaneous coronary intervention (PCI). Although the pivotal trials that led to the approval of DES only enrolled low- to moderate-risk patients with de novo lesions in native coronary arteries1-6, 50% to 80% of patients treated in daily practice fall outside the approved indications of DES7-15. Therefore, extrapolating the results of the pivotal DES studies to this higher risk patient population is problematic. Many important questions concerning performance, efficacy and long-term safety of DES in various patient and lesion subsets remain unanswered. This is especially relevant with respect to stent thrombosis, as the rates are higher in real-world patients than in those from pivotal trials16-18.
The Nobori stent system is a new generation DES incorporating a biodegradable polymer containing the anti-proliferative drug Biolimus A9™ that is only coated on the abluminal side. The results from pivotal randomised trials have shown the very good safety and efficacy profiles of the stent6,19,20.
The aim of the present study was to validate, in daily practice, the performance of the Nobori DES system previously observed in randomised trials.
Materials and methods
Study design and patient population
NOBORI 2 is a prospective, open-label, single arm, multicentre study conducted in 125 centres in Europe and Asia (supplementary appendix) designed to validate, in a real-world setting, the performance of the Nobori DES system. Between April 2008 and March 2009, 3,067 patients were enrolled in the study. The only exclusion criterion was patient’s refusal or inability to provide written informed consent. All patients that had at least one Nobori DES implanted or attempted were included in the analysis.
The study was conducted in accordance with the Declaration of Helsinki and country-specific regulatory requirements. All patients signed informed consent form reviewed and approved by the Institutional Review Board or Ethics Committee of each participating centres.
The Nobori biolimus A9-eluting stent
The Nobori DES system (Terumo Corporation, Tokyo, Japan) comprises four components: the bare metal stent platform, the delivery catheter, biodegradable drug carrier (polylactic acid), and an anti-proliferative substance, Biolimus A9™ (Biosensors International Ltd., Singapore). Contrary to some other DES, the drug polymer matrix is applied only abluminally (toward the vessel wall). The design of the Nobori DES system has been described in details previously6. The Nobori stent was available in lengths from 8 mm to 28 mm and in diameters of 2.5 mm, 3.0 mm and 3.5 mm.
Study procedures
The procedures, and pre- and post-procedure cardiac enzymes, electrocardiogram and medication regimens (aspirin, clopidogrel, ticlopidine) were according to routine hospital practice. Based on preprocedural clinical and angiographic characteristics, the patients were automatically allocated in one of two groups: the on-label group (825 patients with characteristics similar to those of pivotal trials and approved indications) or the off-label group (2,242 patients with acute myocardial infarction, ostial and lesions at bifurcation, left main, saphenous vein graft, lesion containing thrombus, in-stent restenosis, total chronic occlusion.). All baseline and stent implantation angiograms were analysed by an independent core laboratory (CorExperts, Belgrade, Serbia) using Medis software (Medis, Leiden, The Netherlands).
Follow-up
Follow-up (hospital visit or telephone assessment) was scheduled at 1, 6, and 12 months, and yearly thereafter for five years. Clinical evaluations included changes in anginal status, medical therapy and occurrence of adverse events. Angiographic follow-up was not required.
Endpoints and definitions
The primary endpoint was Academic Research Consortium (ARC) defined, device oriented endpoint, a composite of cardiac death, myocardial infarction (MI) (Q-wave and non-Q-wave not clearly attributable to a non-target vessel) and target lesion revascularisation (TLR), also known as target lesion failure (TLF) at 12-month follow-up21. Secondary endpoints were measured at 1, 6, and 12 months and yearly thereafter for five years. These included: 1) TLF, 2) major adverse cardiac events (MACE) defined as cardiac death, MI, or any clinically-driven target vessel revascularisation (TVR), 3) death and MI, 4) TLR and TVR, 5) ARC defined, patient oriented composite endpoint (POCE) that included any death, any MI and any coronary revascularisation 6) stent thrombosis according to ARC definitions. The other endpoints were those of the Nobori pivotal trial6.
Study management
Data were collected on standardised electronic case report forms (KIKA Medical, Paris, France) and stored in a central database. The study monitoring plan included 100% on-line data monitoring and up to 30% of randomly selected patients with 100% on-site source document verification. All site-reported adjudicable adverse events were reviewed and source-verified. In addition, sites with low rates of reported events received additional monitoring visits to confirm adequacy of events reporting. A total of 4,310 queries were generated during on-line monitoring with a resolution rate exceeding 92%. An independent clinical event committee composed of interventional cardiologists not associated with the study (supplementary appendix) reviewed and adjudicated all deaths, MIs, revascularisations and stent thromboses. The angiograms of adverse events were analysed by an independent core laboratory (Mediolanum Cardio Research, Milan, Italy). The study design and scientific management were under the supervision of the executive operational committee and the steering committee composed of cardiologists either participating or not participating in the study (Online appendix).
Statistical analysis and sample size calculation
Baseline characteristics are expressed as mean ±standard deviation, median and range (continuous variables) or frequencies and percentages (discrete variables). Comparisons between groups were performed using a one-way analysis of variance (ANOVA) for continuous variables and a Fisher’s exact test for discrete variables. Kaplan-Meier estimates for device- and patient-related composite endpoints were analysed with the log-rank test. A probability value < 0.05 was considered statistically significant.
NOBORI 2 is a single arm registry and sample size calculation was based on performance goal related to late stent thrombosis. The late stent thrombosis rate is estimated to be ±0.6% from the historical outcome of first generation DES16. The number of patients necessary to estimate the 95% confidence interval of ±0.3% based on an estimated late stent thrombosis rate of 0.6%, is calculated as more than 2,546 patients. Applying these assumptions and taking the attrition rate during the follow-up period into account, the total sample size was set at 3,000 patients. The number of patients is calculated using the following formula:
n=1.96*1.96*p*(1-p)/(CI*CI)
p: incidence rate
CI : the half of the width of 95% confidence interval
Data analysis was performed by an independent statistical office (SBD ANALYTICS, Bekkevoort, Belgium).
Results
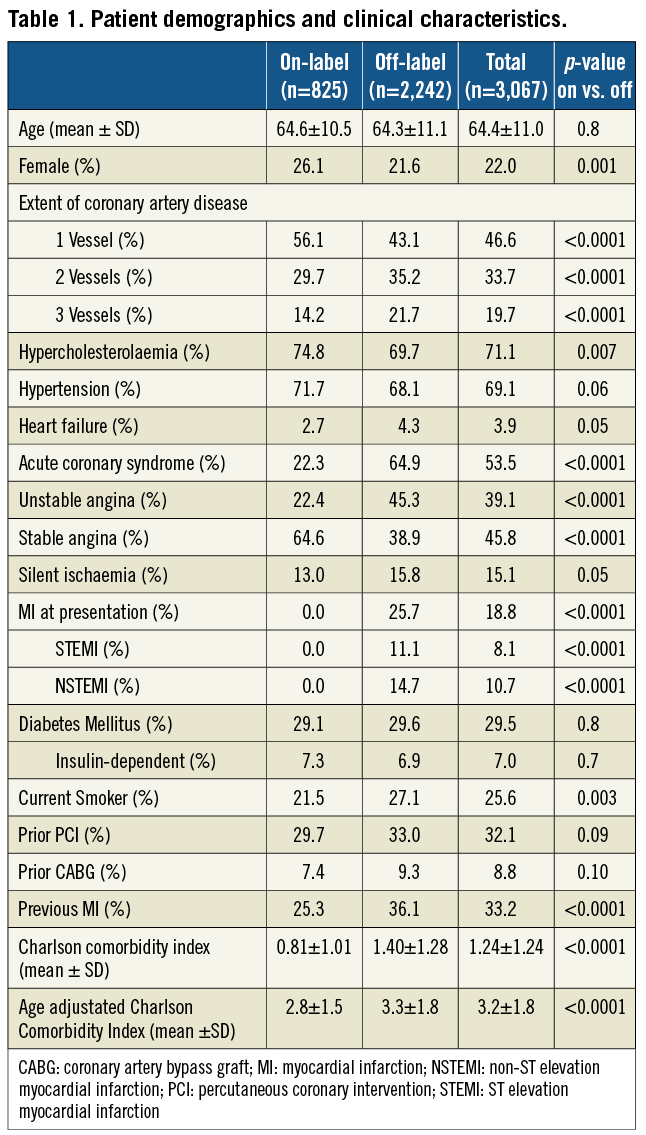
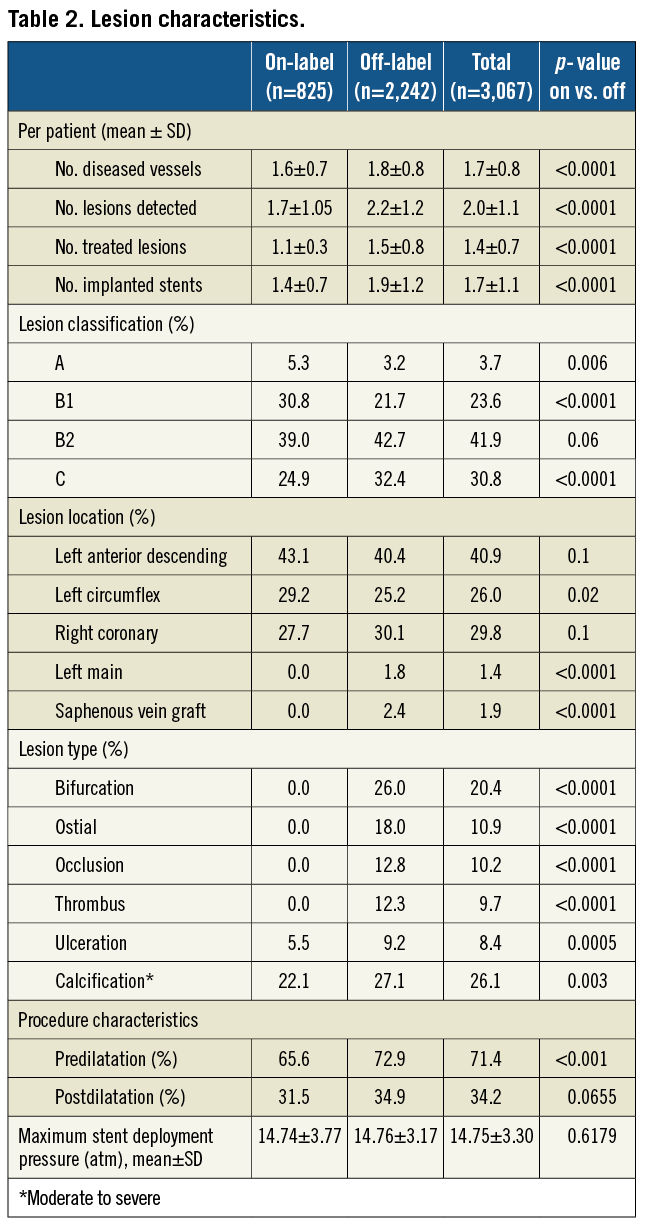
A total of 3,067 consecutives patients with 5,463 lesions were enrolled in the study. Overall, the mean age was 64.4±11.0 years and 78.0% were men. Two- or three-vessel disease was present in 53.4% of patients, 53.5% had acute coronary syndrome and 29.5% had diabetes (insulin-dependent in 7.0%). The lesions were classified B2/C in 72.7%, 20.4% were in bifurcations and 26.1% had moderate to severe calcification. Of the 3,067 patients, 2,242 (73%) were treated for at least one condition outside of the approved indications. Besides the greater lesions complexity, which is representative of contemporary practice, patients in the off-label group had significantly higher mean Charlson comorbidity index scores and a particularly higher prevalence of renal disease22. Patient demographics, clinical and lesion characteristics are presented in Table 1 and Table 2.
Procedural characteristics
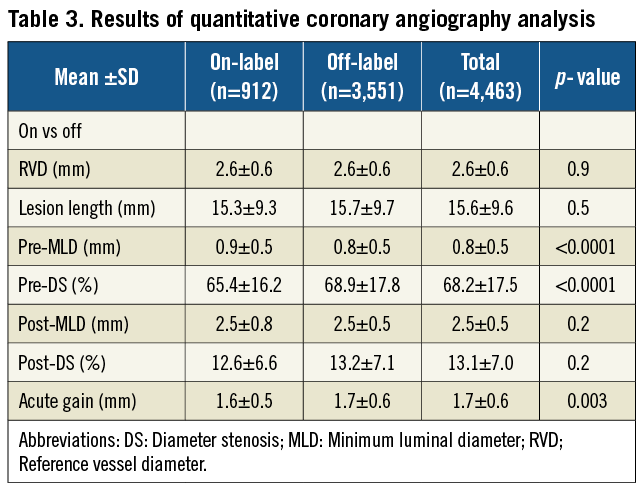
Nobori DES was successfully deployed in 98.8% of the lesions. Eighty-two percent (82.4%) of patients were treated with Nobori DES alone, 17.2% with mixed stents and 0.5% with non-study stents. Irrespective of the stent type, the complexity of lesions was similar across all stents (data not shown). The main reasons for the implantation of non-study stents were: reimbursement regulations (6.0%); size availability (7.0%); investigators’ preference to treat additional lesions with other DES or bare-metal stents (2.0%) and Nobori DES failure (0.5%). Pre-dilatation was performed more often in the off-label group, but stent deployment pressure and post-dilatation frequency were similar (Table 2). The mean reference vessel diameter and lesion length were representative of currently recommended indications for the use of DES (Table 3).
Safety outcomes
Twelve- and 24-month follow-up was available in 97% and 95% of the patient population. The cumulative rates of the TLF, MACE and its individual components are presented in Table 4. Compared with patients treated according to the approved indications, the primary endpoint of TLF was higher in the off-label group at both follow-up time points, with similar increase between two years (from 2.2% at one to 2.8% at two years and from 4.5% to 5.9% in on- and off-label, respectively). When analysed according to stent type, patients treated with the Nobori DES alone at two years had a significantly lower rate of TLF than those treated with mixed stents (4.9% vs. 5.7%, p=0.003). Eighty-six patients (2.8%) died during follow-up, with 49 (1.6%) deaths deemed cardiac in origin. Rates of MI at two years were higher in off-label group (61 or 2.7% vs. 12 or 1.5%), the majority (82%) were non-Q-wave and 64% were target vessel-related. The 24-month Kaplan-Meier estimates of the composite endpoints are presented in Figure 1 and Figure 2.
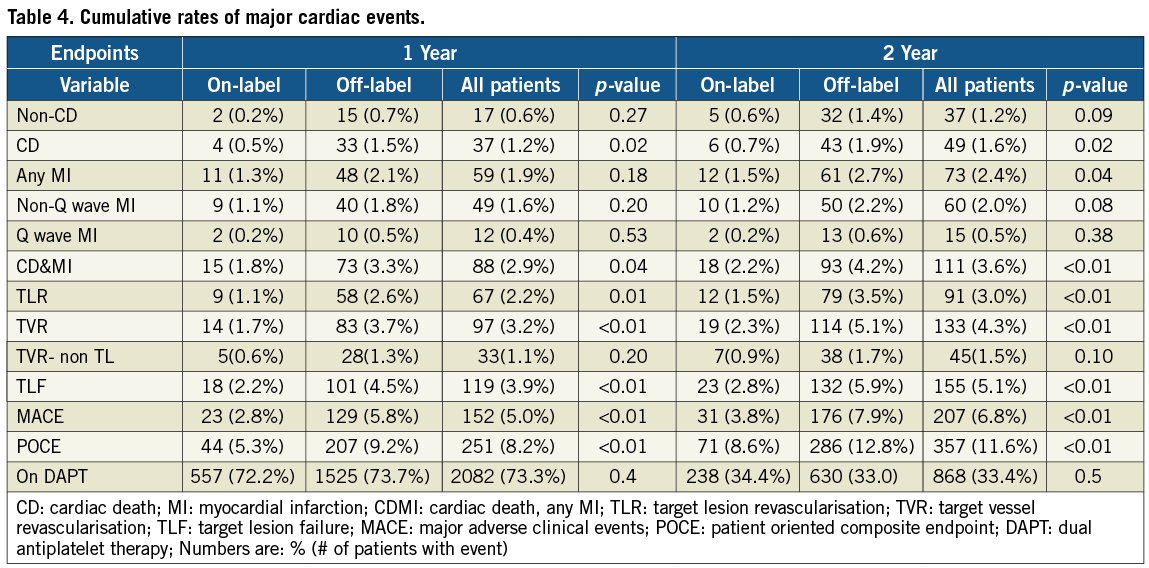
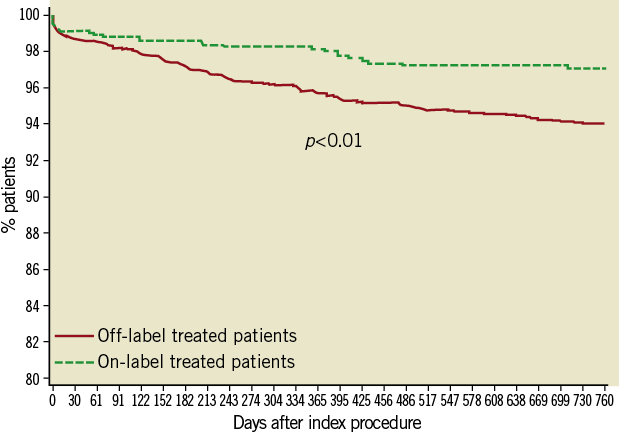
Figure 1. Kaplan-Meier survival curve - Target lesion failure.
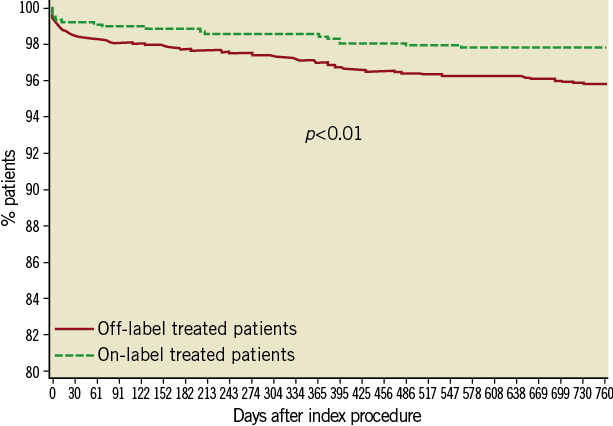
Figure 2. Kaplan-Meier survival curve - Cardiac death and myocardial infarction.
According to the ARC definitions, 25 (0.8%) cases of definite and probable, and 14 (0.5%) cases of possible stent thromboses were reported during 2-year follow-up. Rates of stent thrombosis were similar between groups, and most of the events were subacute (Table 5). Four of the patients experiencing late or very late ST were still on dual antiplatelet therapy at the time of event, while three patients were off the therapy.
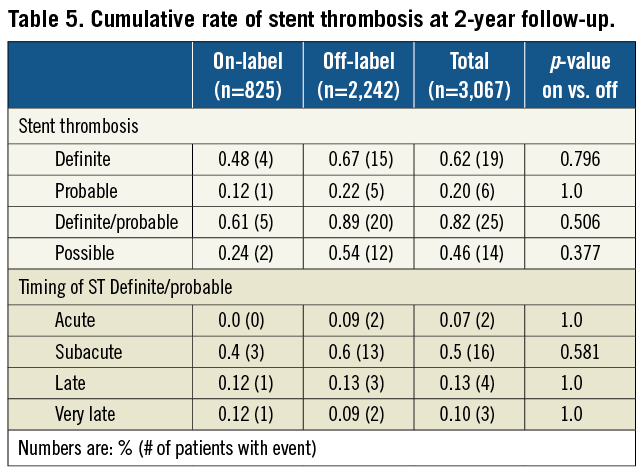
Observed rate of late ST in NOBORI 2 trial was 0.13% (exact 95% CI Clopper-Pearson=0.04%-0.33%). The superior bound of the 95%CI was 0.33%, showing that the value of the late stent thrombosis rate up to 12 months in the study population lies in an interval bellow the assumed 0.6%, that is generally accepted rate for the first generation DES.
Bleeding or vascular complications remained stable at 1.3% throughout follow-up, with no new bleeding events between year one and two.
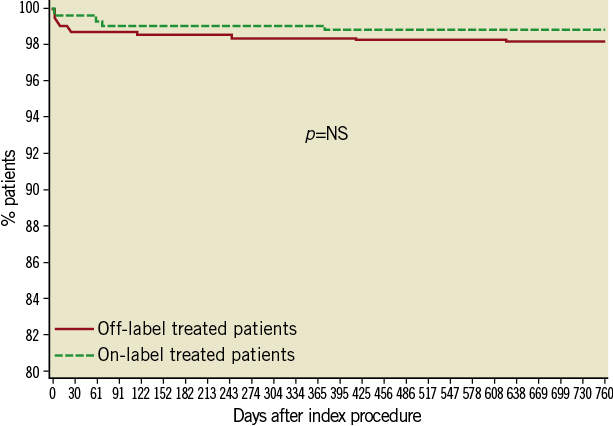
Figure 3. Kaplan-Meier survival curve - Stent thrombosis (definite and probable).
Efficacy outcomes at one year
Target lesion revascularisation at one year was reported in 67 (2.2%) patients and was more frequent in the off-label group (2.6% vs. 1.1%, p=0.01) (Table 4). Target vessel revascularisation (non-TLR) was reported in 33 (1.1%) of patients, with rates similar between groups (0.6% vs. 1.3%, p=0.2).
Efficacy outcomes at two years
Between one and two years, 24 more patients underwent target lesion revascularisation reaching 91 (3.0%) patients. The frequency in the off-label group continued to be higher (3.5% vs. 1.5%, p=<0.01) (Table 4 and Figure 4). Target vessel revascularisation (non-TLR) at two years was reported in 45 (1.5%) patients (1.7% vs. 0.9% in off-label and on-label groups, respectively, p=0.09).
The anginal status of patients in both groups remained significantly improved, with 88% of patients not experiencing any symptom at one and two years after the procedure.
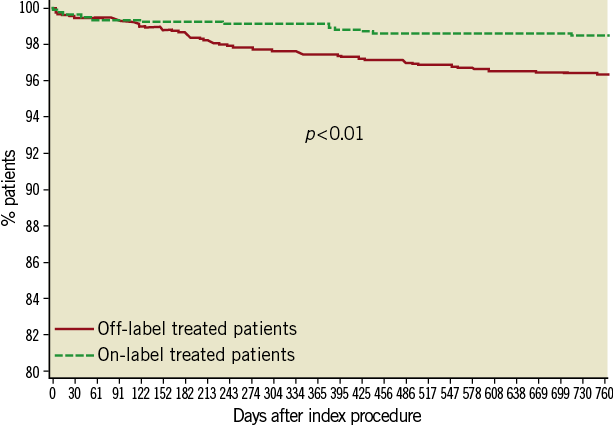
Figure 4. Kaplan-Meier survival curve - Target lesion revascularisation.
Discussion
The NOBORI 2 study was designed to further validate the safety and efficacy of the Nobori DES system for the treatment of coronary artery lesions. With 73% of patients presenting with ≥1 off-label indications, this is the first study to evaluate the use of the Nobori DES in a real-world setting, and the largest to date using a stent with a biodegradable polymer. A very high rate of source data verification, as mandated by regulatory authorities, represents one of the major strengths of this registry.
The main findings of this 24-month clinical follow-up are 1) the rates of TLF, cardiac death and MI were low and, as expected, higher in more complex patients and lesions, 2) stent thromboses were rare and similar between groups, with 72% of events categorised as acute or subacute and 3) the rates of MACE for on-label-treated patients were similar to those reported in pivotal studies. Overall, these findings suggest the good clinical performance of the Nobori DES system in a real-world population with a large proportion of high-risk patients.
Long-term follow-up of patients treated for more complex lesions has revealed higher rates of adverse events, particularly stent thrombosis8,14. It has been suggested that the permanent polymer containing the antiproliferative drug was partly responsible for delayed vascular healing, thus a possible substrate for stent thrombosis23,24. Because of these limitations, new generation DES have been developed using biodegradable polymers6,15. The Nobori DES system employs a matrix of biodegradable polymer carrier (poly-lactic acid) and the antiproliferative drug Biolimus A9™ applied only to the abluminal surface of the stent platform. By reducing contact with the blood, the abluminal coating might enhance the attachment of endothelial progenitor cells from the peripheral circulation or in-growth of endothelial tissue from the distal and proximal edges of the stent20. It has been reported that endothelium-dependent vasomotion at adjacent stent segments was better preserved in vessels treated with Nobori DES than with first generation DES25.
Our results are in agreement with previous studies of the Nobori DES6,19,20. For patients treated according to approved indications (on-label), the 24-month rates of cardiac death, MI, TLR and stent thrombosis were 0.7%, 1.5%, 1.5% and 0.6%, respectively, and similar to the 0.7%, 3.3%, 0.0% and 0.0% rates reported in the pivotal randomised NOBORI 1 trial6. Our results also extend the initial findings to the general population, as 73% of patients were treated for off-label indications and the majority had a high disease burden, and complex lesions and medical conditions at presentation. The rates of adverse events we observed compare favourably with those of other DES studies that enrolled patients without major exclusion criteria8,12-15,27-30. The safety and efficacy profiles of the Nobori DES closely resemble those of XIENCE V™ (Abbott Vascular, Abbott Park, IL, USA) from the COMPARE trial that enrolled similar patient population27. The reported composite rate of cardiac death, MI and TLR of 7.4% at 2 years, is similar to 6.8% reported in our study. The results of XIENCE V USA registry are also similar to our findings indicating that new generation DES might have similar benefits as compared to first generation of those devices28.
It is generally accepted that use of DES for off-label indications is associated with higher rates of stent thrombosis16-18. In the present study, the two-year cumulative rate of definite and probable stent thromboses was relatively low at 0.8%, with the majority of events categorised as subacute. In fact, it was either similar or lower than those reported in randomised trials and registries involving so-called all-comer population, with rates ranging from 0.7% to 2.6%13,15,27 at one year. Noteworthy is the fact that some of these trials13,15 treated more patients in the course of an evolving MI which might have impacted the overall rate of stent thrombosis.
Recently reported long term (4-year) data from LEADERS trial found that BioMatrix™ stent (Biosensors International Ltd.) with biodegradable polymer showed 80% relative risk reduction of very late stent thrombosis (1-4 years) when compared to first generation durable polymer DES31. In our study Nobori DES also showed very low rate of late and very late stent thrombosis adding further evidence to the hypothesis that biodegradable polymer might improve long term safety of DES. To fully assess this hypothesis a randomised trial, sufficiently powered to detect difference in such rare events as stent thrombosis, would be needed.
Study limitations
There are several limitations to our study. First, it was not a randomised study and, as such, limits the possibility of direct comparisons with other DES. Second, as with every registry, a certain level of under-reporting could exist. However, due to a meticulous on-line and on-site monitoring and data source verification, we believe that most of the relevant events were either directly reported by the investigators, detected during monitoring or triggered from the database. The only exception could be periprocedural MIs as approximately 20% of patients did not have post-procedure cardiac enzymes measurements. Nevertheless, we believe that very high 12- and 24-month follow-up compliance rate and the high number of non-MACE adverse events reported by the investigators indicate high reliability of the current data. Third, because enrolment of consecutive patients was recommended but not verified, it is possible that this pattern was not strictly followed. Fourth, the two-year follow-up may not fully confirm the safety of the stent in high-risk patients. The planned five-year follow-up will therefore be of significant interest, especially since there are good theoretical reasons to believe that a biodegradable polymer may confer better long-term results in terms of stent thrombosis.
Conclusions
In summary, besides confirming the excellent short- and long-term safety and efficacy of the Nobori DES that were initially documented in randomised pivotal trials, the present series demonstrates promising results for the treatment of patients with high-risk clinical features and more complex lesion anatomy. As in contemporary clinical practice the majority of patients are treated for one or more conditions falling outside the approved indications of many DES, our results provide reassurance as to the safety of such treatment.
Acknowledgments
We thank Danny Detiège, Claude Dragos, Virginie De Hemptinne, Anna Ramazzotti, Irene Barriocanal, Goran Babic (Terumo Europe) and Danielle Libersan for assistance in study design and management or manuscript preparation, and Erik Spaepen (SDB Analytics) for statistical analysis.
Funding
The study was funded by Terumo Corporation, Tokyo, Japan and Terumo Europe, Leuven, Belgium.
Conflict of interest statement
D. Paunovic is an employee of Terumo Europe N.V. The other authors have no conflict of interest to declare in relation to the content of this manuscript except serving as principal investigator, steering committee members or investigators of this study.
Online data supplement

