Abstract
Aims: To assess the safety and performance of the Nobori drug-eluting stent with biodegradable polymer versus the TAXUS drug-eluting stent with permanent polymer, in the treatment of patients with de novo coronary artery lesions.
Methods and results: NOBORI 1 was a multicentre, randomised (2:1), prospective, controlled, clinical trial which enrolled 363 patients (238 Nobori and 125 TAXUS) with up to two de novo lesions in two epicardial vessels. The primary endpoint was in-stent late loss at nine months, while secondary endpoints included safety and efficacy up to five years. At five years, clinical data were available for 350 patients (96%). There were no differences in the composite of death and myocardial infarction (10.9% vs. 11.2%) and target lesion failure (9.2% and 10.4%), while ischaemia- and non-ischaemia-driven target lesion revascularisations were less frequent in the Nobori (6.3%) than in the TAXUS arm (16.0%). The rates of stent thrombosis (definite and probable according to the ARC definitions) were 0.0% and 3.2%, in the Nobori and TAXUS stents, respectively (p=0.014).
Conclusions: Five years after implantation, the Nobori DES resulted in durable treatment effects with very low TLR and no stent thrombosis. The study was not powered to assess the differences in clinical endpoints. These data are hypothesis-generating.
Introduction
Drug-eluting stents (DES) with biodegradable polymers were developed as an answer to the potential long-term undesirable effects of permanent polymers. Nobori® (Terumo Corp., Tokyo, Japan) is a DES coated with the matrix of Biolimus A9™ (Biosensors International, Singapore) and biodegradable polymer (polylactic acid) that was shown in animal studies to degrade completely within nine to 12 months post implantation. The available clinical results of the Nobori DES indicate good efficacy and safety, with particularly low rates of late and very late stent thrombosis across a variety of interventional practices and patient populations1-4.
NOBORI 1 was a prospective, multicentre, controlled clinical trial which randomised (2:1) 363 patients to either Nobori DES or TAXUS Express™/Liberté™ DES (Boston Scientific, Natick, MA, USA). In this study, treatment with Nobori DES was associated with a significant reduction of in-stent and in-segment late loss at nine months post implantation1,2. The NOBORI 1 clinical trial was not powered to detect differences in clinical outcomes but, considering the relevance of long-term treatment, we have decided to report five-year follow-up findings.
Materials and methods
The enrolment of patients in the NOBORI 1 study was conducted in two phases. After enrolment of the first 120 patients (85 Nobori and 35 TAXUS Express) the study was temporarily suspended and resumed by replacing TAXUS Express, which was discontinued in the European market, with TAXUS Liberté. Phase 2 enrolled 243 patients (158 Nobori and 85 TAXUS Liberté).
The study design and nine-month results for phase 1 and phase 2 of the NOBORI 1 clinical trial have already been reported1,2. In summary, NOBORI 1 is a multicentre, randomised (2:1), prospective, controlled study which enrolled 363 patients with up to two de novo lesions in two epicardial vessels. All events were adjudicated by an independent clinical events committee, and the study was extensively monitored by an independent clinical research organisation (Cardialysis, Rotterdam, The Netherlands) which was responsible for data management and statistical analysis as well as for angiographic and IVUS assessments.
Endpoints
The primary endpoint for which the study was powered was in-stent late loss at nine months. Clinical endpoints assessed at one, six, nine and 12 months and yearly up to five years were reported earlier. However, after the publication of the Academic Research Consortium (ARC) definition of endpoints, the protocol was amended to accommodate new definitions and included target lesion failure (TLF), a device-oriented composite endpoint (cardiac death and MI not clearly attributed to non-target vessel and clinically indicated target lesion revascularisation), and a patient-oriented composite endpoint of any death, any MI and any coronary revascularisation. Stent thromboses were also adjudicated according to protocol and according to ARC5.
Statistical methods
All trial endpoints, including the primary endpoint, were analysed on the intention-to-treat population. Angiographic endpoints were analysed on a per lesion basis. Clinical events including death and MI are reported on a per patient basis while revascularisation is reported per lesion treated. For continuous variables, differences between the treatment groups were examined by analysis of variance, while the Fisher’s exact test was used for categorical variables. Furthermore, due to the significant difference in representation of patients with diabetes mellitus in the two groups, a sub-analysis of patients with this condition has also been carried out. All statistical analyses were performed using SAS statistical software, version 9 (SAS Institute Inc., Cary, NC, USA).
Results
At five years, clinical information was available for 350 patients (96%), 231 (97%) in the Nobori and 119 (95%) in the TAXUS arm. Twelve patients were lost to follow-up and one patient refused further participation (Figure 1).

Figure 1. NOBORI 1 - study flow chart.
Baseline demographic data
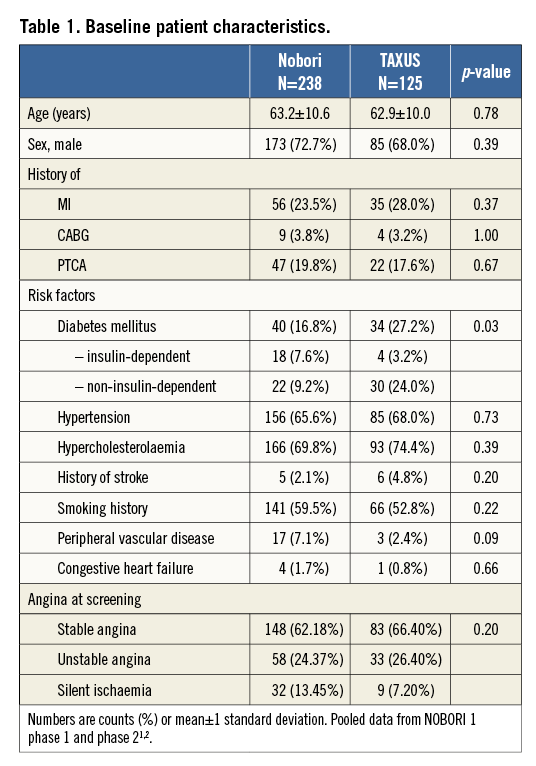
Baseline demographic characteristics of the NOBORI 1 trial (both phases combined) are presented in Table 1, showing comparable characteristics in the two treatment arms, except a higher incidence of diabetes mellitus in the TAXUS arm and insulin-dependent diabetes mellitus in the Nobori arm. Angiographic data at baseline were well matched (data published earlier)1,2.
Clinical outcomes
Five-year clinical outcomes are shown in Table 2. Up to five years in the Nobori arm, 12 patients died from cardiac causes. The clear circumstances of death were not available for all patients, but they were adjudicated as cardiac in line with protocol definitions. According to the investigator’s judgement, a possible relation to the study device was noted in only two cases. Thirty percent of patients who died from cardiac causes had diabetes mellitus and the average age at enrolment was 79 years. In the TAXUS arm, there were three cardiac deaths, two of which were related to stent thrombosis, and a clear relationship to the study device was established.
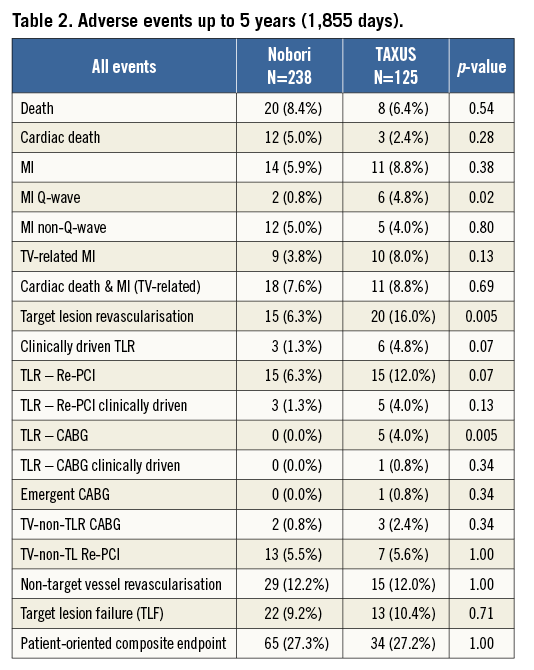
The rate of target lesion failure was similar between the groups (9.2% in the Nobori arm and 10.4% in the TAXUS arm). The frequency of a patient-oriented composite endpoint was also similar.
There was a trend towards lower MI (5.9% vs. 8.8%) and a significant difference in the rate of Q-wave MI (0.8% vs. 4.8%, p=0.02) in favour of the Nobori DES. The total rate of ischaemia- and non-ischaemia-driven TLR was also significantly lower in the Nobori arm (6.3% vs. 16.0%, p=0.005).
Most of the non-ischaemia-driven TLRs occurred at the time of angiographic follow-up at nine months.
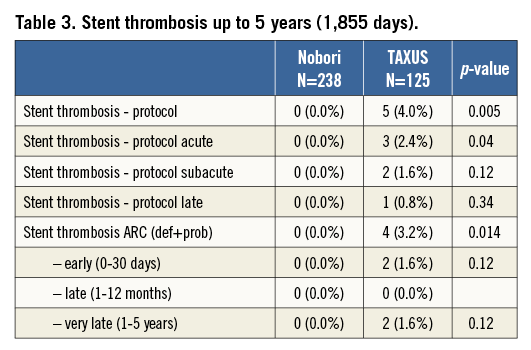
In the Nobori DES there were no definite and probable stent thromboses (0.0%), while there were four (3.2%) thromboses detected in the TAXUS stent (Table 3). This difference was statistically significant (p=0.014).
The clinical outcomes in the diabetic subset of patients were similar to the total population with TLF of 10.0% and 12.0% in the Nobori and TAXUS arms, respectively. There were no TLRs (0.0%) in the Nobori arm while in the TAXUS arm the rate was 8.8%. Two patients (5.9%) in the TAXUS arm and none (0.0%) in the Nobori arm suffered stent thrombosis (data not shown).
Discussion
Our study represents the longest available follow-up of the Nobori DES and thus provides essential data on the long-term efficacy and safety of this stent. The NOBORI 1 clinical trial was designed to prove the non-inferiority of the Nobori DES versus the TAXUS DES for angiographic in-stent late lumen loss at nine months post procedure. Therefore, data and results of clinical endpoints are for information only. The study was not powered to detect clinical differences.
The principal findings of the five-year analysis of the NOBORI 1 clinical trial are the following. 1) The Nobori stent is both safe and effective when used for the treatment of patients with coronary artery disease with characteristics as per the eligibility criteria for the NOBORI 1 clinical trial. 2) Up to five years, 6.3% of patients underwent target lesion revascularisation in the Nobori arm versus 16.0% in the TAXUS arm (p=0.005), the majority being non-ischaemia-driven. 3) The rates of death and MI were similar in the two treatment arms. 4) There were no stent thromboses (definite and probable) in Nobori DES, while there were four (3.2%) in TAXUS DES (p=0.01).
At five years, post-procedure clinical follow-up was available for 96% of patients in both arms. This high follow-up compliance rate is important testimony to the quality of the study and acceptability of its results. Target lesion failure, a composite endpoint which is recommended for comparison between the two stents, had a similar rate in the two arms at five years. Long-term clinical outcomes are considered to be more associated with patient’s comorbidities, age and progression of the underlying disease rather than to the stent itself6. The two critical patterns reported for DES, i.e., late catch-up with increased TLRs and very late stent thrombosis, were both absent in the patients treated with Nobori DES7-9.
The frequency of patients still on dual antiplatelet therapy at five years was 16%, mainly as a consequence of repeated revascularisations. Cardiac death in the Nobori arm tended to be higher but the difference was not significant. The small number of patients in this trial does not allow any conclusion in this regard and could be a play of chance. Furthermore, the difficulty of collecting detailed evidence about circumstances of death five years after treatment leaves no option to the clinical events committee other than to adjudicate an event as cardiac death.
Overall, results of Nobori DES in the NOBORI 1 clinical trial are comparable to the results of other second-generation DES in pivotal trials which enrolled similar patient populations10-14. In the SPIRIT III trial, which compared XIENCE V® DES (Abbott Vascular, Santa Clara, CA, USA) with TAXUS Express DES, the TLF rate of 12.9% in the XIENCE arm was similar to 9.2% in the Nobori arm, but the difference was much higher in the TAXUS arm (10.4% in NOBORI 1 versus 19.0% in SPIRIT III). This discrepancy could potentially be related to differences between TAXUS Express and TAXUS Liberté, which was used for treatment of the majority of patients in the NOBORI 1 trial.
Looking specifically at the most relevant clinical measure of efficacy, clinically indicated TLR, the rate in the Nobori DES was rather low and, at 1.3% at five years, is one of the lowest reported. In SPIRIT III, the rates were 8.9% and 12.9% in the XIENCE and TAXUS arms, respectively10. In TAXUS IV, it was 9.1% in the TAXUS arm, while in ENDEAVOR III the rates were 8.1% in the Endeavor arm and 6.5% in the CYPHER arm14,12. In most pivotal clinical trials of DES, mandatory angiographic follow-up triggered a higher number of revascularisations in the first year, making it difficult to assess the phenomenon of late catch-up which has been reported for some DES7. In our study, we did not observe such a trend in either of the study arms.
Stent thrombosis, one of the most critical events associated with the first-generation DES8,9, was not recorded in any of the patients in the Nobori arm, while there were four patients (3.2%) in the TAXUS arm who suffered from stent thrombosis (definite and probable according to ARC definitions). Two of the patients developed very late stent thrombosis.
In other similar studies the rate of ST was also relatively low, reaching 1.5% and 1.9% in the XIENCE and TAXUS arms, respectively, in the SPIRIT III trial10, 2.2% and 2.1% in TAXUS and bare metal stents, respectively, in TAXUS IV14, and 0.7% and 0.9% in Endeavor® (Medtronic, Minneapolis, MN, USA) and CYPHER® (Cordis, Johnson & Johnson, Warren, NJ, USA) stents, respectively, in the ENDEAVOR III trial12.
Even though NOBORI 1 was a randomised study, there were significant differences in the rate of diabetes mellitus. In the TAXUS arm there were more patients with this comorbidity, while in the Nobori arm there were more patients with insulin-dependent diabetes mellitus. The post hoc sub-analysis of these patients showed very similar results to the overall population with no differences in the two treatment arms.
DES with biodegradable polymer were designed to improve long-term safety, which was the major concern with the first-generation devices7-9. The only study that compared these two types of stent which was powered for a clinical endpoint was the LEADERS trial15. In this trial, the BioMatrix™ (Biosensors, Morges, Switzerland) stent, which is very similar to the Nobori stent, showed significantly lower late and very late stent thrombosis rates when compared to the CYPHER stent (0.66% vs. 2.5%; p=0.003). In our study, we also observed a significantly lower rate of stent thrombosis in the Nobori arm than in the TAXUS arm (0.0% vs. 3.2%; p=0.01). These findings indicate better long-term safety of DES with bioresorbable polymer versus first-generation DES. The clinical outcomes of randomised studies between DES with biodegradable polymer and new-generation permanent polymer DES did not show similar differences in the short and medium term, and long-term results of several ongoing large trials are awaited to elucidate the value of biodegradable technology in contemporary PCI practice.
Study limitations
NOBORI 1 was powered for non-inferiority of the Nobori versus TAXUS DES for the angiographic endpoint of in-stent late loss at nine-month follow-up. The results of the present analysis should therefore be considered hypothesis-generating. The comparator in this study was TAXUS Express in phase 1 and TAXUS Liberté in phase 2; however, both stents showed similar outcomes.
Conclusions
The five-year clinical follow-up of the NOBORI 1 trial demonstrated the sustained long-term safety and efficacy of the Nobori DES. The low frequency of clinically indicated TLR and the absence of stent thrombosis indicate that the specific design of the Nobori DES, incorporating an antiproliferative limus drug and a biodegradable polymer coated only on the abluminal side, may offer long-term benefits.
| Impact on daily practice The NOBORI 1 trial was not powered to assess differences in clinical endpoints. However, the lower rate of TLR and ST is reassuring in terms of the safety and efficacy profile of this biodegradable polymer drug-eluting stent. These long-term results confirm previous data showing some superiority to first-generation DES coming from both randomised trials and large registries, and support its safe use in daily practice. |
Appendix. The NOBORI 1 Clinical Investigators
Australia: Stephen Worthley, Royal Adelaide Hospital, Adelaide; Ian Meredith, Monash Medical Centre, Clayton, Victoria; Belgium: William Wijns, Onze-Lieve-Vrouwziekenhuis, Aalst; Victor Legrand, Centre Hospitalier Universitaire de Liège, Liège; Marc Castadot, Clinique St-Jean, Brussels; Denmark: Leif Thuesen, Skejby Sygehus, Aarhus; France: Bernard Chevalier, Centre Cardiologique du Nord, Paris; Jean Marco, Clinique Pasteur, Toulouse; Emmanuel Teiger, Hôpital Henri Mondor, Créteil; Marie-Claude Morice, Institut Jacques Cartier ICPS, Massy; Martial Hamon, Centre Hospitalier Universitaire Caen, Caen; Philippe Garot, Hôpital Claude Gallien, Quincy; Didier Carrie, Centre Hospitalier Universitaire Rangeuil, Toulouse; Germany: Nicolaus Reifart, Kardiologische Praxis Professor Reifart & Partner, Bad Soden; Sigmund Silber, Kardiologische Gemeinschaftspraxis und HKL, München; Christian Hamm, Kerckhoff Klinik GmbH, Bad Nauheim; Bernd Nowak, Bethanien Krankenhaus, CCB, Frankfurt; Thomas Schiele, Klinikum Innenstadt der LMU, München; Gerhard Schuler, Universitätsklinikum Leipzig/Herzzentrum, Leipzig; Karl Hauptmann, Krankenhaus der Barmherzigen Brüder, Trier; Korea: Seung-Jung Park, Asan Medical Center, Seoul; The Netherlands: J.J. Bonnier, Catharina Ziekenhuis, Eindhoven; Harry Suryapranata, Isala Clinic, Zwolle; Patrick Serruys, Erasmus MC, Thoraxcentrum, Rotterdam; Spain: Eulogio Garcia, Gregorio Marañón, Madrid; Antoni Serra, Hospital del Mar, Barcelona; United Kingdom: Martyn Thomas, King’s College Hospital, London; F. Fath-Ordoubadi, Royal Infirmary, Manchester; David Hildick-Smith, Royal Sussex County Hospital, Brighton.
Guest Editor
This paper was guest edited by Ron Waksman, MD, Interventional Cardiology, MedStar Washington Hospital Center, Washington, DC, USA.
Conflict of interest statement
B. Chevalier receives consulting fees from Terumo. D. Paunovic is an employee of Terumo, sponsor of the study. The other authors have no conflicts of interest to declare. The Guest Editor reports personal fees from Biotronik, personal fees from Medtronic, grants and personal fees from AstraZeneca, grants and personal fees from Boston Scientific, personal fees from Abbott Vascular, grants from The Medicines Company, and grants from Edwards Lifesciences.

