Abstract
Aims: The aim of this multicentre, non-randomised trial was to evaluate the safety and efficacy at 180±14 days of a pimecrolimus eluting coronary stent based on a cobalt-chromium platform and a poly-L-lactic acid (PLLA) bioresorbable polymer.
Methods and results: Sixty-one patients, with single de novo coronary lesions <14 mm in length and a reference vessel diameter of 3.0 to 3.5 mm, were enrolled in five centres (Germany and Belgium). Angiography and IVUS were performed at baseline, post-procedure and 180±14 days later. The primary endpoint was a composite of major adverse cardiac events (MACE) at 180±14 days and expected to be below 20%. Patients had single vessel disease in 59%, 2-vessel disease in 28% and 3-vessel disease in 13% of cases. MACE rate at 180±14 days was 18.0%. Binary in-stent restenosis was 32.7% due to in-stent late lumen loss of 1.11±0.65 mm by QCA. Stent thrombosis rate at 30±7 and 180±14 days was 1.6% and 3.3%, respectively. Overall TLR rate at 30±7, 180±14 days and 12±1 months was 1.6%, 27.9% and 32.8% respectively.
Conclusions: The primary endpoint was met at 180±14 days. However, the anti-restenotic effect of the pimecrolimus eluting stent did not reach levels similar to clinically established DES.
Introduction
Drug eluting stents (DES) are a breakthrough in the prevention and treatment of obstructive atherosclerotic lesions and in-stent restenosis. Potential adverse effects related to a prolonged healing process have been attributed to the elution of non-specific anti-mitotic agents and/or the use of poorly biocompatible durable polymers. The pimecrolimus eluting coronary stent ProGenic (Biotronik AG, Bülach, Switzerland) combines a cobalt-chromium platform with a poly-L-lactic acid (PLLA) bioresorbable polymer and the pimecrolimus drug, a 33-epi-chloro-derivative of the macrolactam ascomycin.
The aim of this multicentre, non-randomised, first-in-man (FIM) study, the ProLimus I trial, was to evaluate the safety and efficacy of a next generation DES that combines a predominantly anti-inflammatory agent with a biodegradable polymer.
Methods
Study population
From December 2006 until June 2007, a cohort of 61 patients with single de novo coronary artery lesions <14 mm in length and a reference vessel diameter of 3.0 to 3.5 mm, and 50-100% diameter stenosis were enrolled in the ProLimus I trial at five centres (Germany and Belgium).
Patients presenting with an acute myocardial infarction within 48 hours of the intended treatment were excluded. Other criteria for exclusion were heavily calcified lesions, ostial lesions or a left ventricular ejection fraction < 30%. A written informed consent was obtained from all the patients prior to enrolment.
Device description
The pimecrolimus eluting coronary stent ProGenic consists of a metal stent structure coated with a pimecrolimus/polymer matrix, mounted on a PTCA balloon delivery system and sterilised by e-beam radiation.
Stent platform
The cobalt-chromium stent platform is a tubular, balloon-expandable stent sculpted by laser from a single tube of L-605 (CoCr) alloy. The stent consists of circular segments at each end followed by a transition zone and helicoidally arranged struts in the middle. Each loop of the helix is connected to the next loop by three longitudinal struts. The bare body stent surface is fully coated with an intermediate layer of amorphous hydrogen-rich silicon carbide (aSiC:H) providing an interface to the polymer coating. This material has semiconducting properties that efficiently prevent the electron transfer from fibrinogen to the metal surface in vitro, through which the conversion from fibrinogen to fibrin, and its deposition at the stent surface, is reduced. Additionally, a SiC:H-coated stents exhibit a lower adhesion and activation of blood platelets and leukocytes.
Polymer coating
The polymer compound used as a carrier material for supply and release of pimecrolimus is a high molecular poly-L-lactic acid (PLLA). It is applied on the entire stent surface as a thin layer.
It is mixed with the drug pimecrolimus in an approximately 55/45 weight % relationship. PLLA is a highly biocompatible, bioresorbable material that is degraded mainly by hydrolysis. Clinical experience using PLA type polymers has been reported with use of the Igaki-Tamai stent (Igaki Medical Planning Co., Kyoto, Japan) and the bioabsorbable everolimus-eluting stent system BVS (Abbott Vascular, Santa Clara, CA, USA). Today, two CE marked DES are using a PLA type polymer as drug carrier, the BioMATRIX™-Stent (Biosensors International, Singapore) and the Nobori™ drug eluting stent system (Terumo Corporation, Tokyo, Japan).
Extensive data on tissue response to PLLA are available from preclinical studies in pig coronaries. PLLA coated BMS without drug showed similar efficacy data in efficacy animal trials and consistently did not provoke any safety issue.
Drug component
The pharmacological agent used in this study is pimecrolimus, a 33-epi-chloro-derivative of the macrolactam ascomycin. The interest in pimecrolimus has been substantial because of its significant anti-inflammatory activity, immunomodulatory activity and its low systemic immunosuppressive potential. Pimecrolimus is a cell-selective inhibitor of the production and release of pro-inflammatory cytokines. Pimecrolimus is not an anti-mitotic compound and, therefore, it is believed not to inhibit re-endothelialisation, the growth of the endothelial cells lining the interior vessel surface and maintaining the healthy function of blood vessels.
The primary mechanism of action of pimecrolimus is the blockage of T cell activation. Both T lymphocytes and neutrophils are involved in restenotic lesion formation. Pimecrolimus (like all ascomycins) is an immunophylin ligand, which binds specifically to the cytosolic receptor, immunophylin macrophylin-12 (FKBP-12). This pimecrolimus-macrophylin complex effectively inhibits the protein phosphatase calcineurin, by preventing calcineurin from dephosphorylating the nuclear factor of activated T cells (NF-AT), a transcription factor. This results in the blockage of signal transduction pathways in T cells and the inhibition of the synthesis of inflammatory cytokines, specifically Th1 (interleukin-2, interferon-γ)- and Th2 (interleukin-4, interleukin-10)-type cytokines. Pimecrolimus has also been shown to prevent the release of cytokines and pro-inflammatory mediators from mast cells. Mast cells and macrophages are involved in the formation of human coronary atherosclerotic plaques.
The target dose represents a total drug weight of 45 µg/mm stent length. Doses below and exceeding this range have been evaluated in previous animal studies at various time points after implantation. Promising safety and efficacy results have been obtained in porcine overstretch coronary models when tested in typical efficacy set up.
Preclinical investigations
Extensive preclinical investigations comprising > 160 ProGenic stent implantations into domestic pigs, miniature pig coronaries and rabbit iliac arteries were carried out. All follow-up analyses at 2, 4, 12, 26 and 52 weeks showed benign tissue responses and low inflammation scores. There was no evidence of any thrombotic or other safety relevant events. A 3-fold higher dosage than used for the clinical trial did not reveal any safety issues. Efficacy endpoints like angiographic stenosis or histomorphometrically assessed neointimal area consistently showed tendency towards more favourable outcome for the pimecrolimus eluting devices. No statistically significant difference was reached in comparison to the CoCr bare stent. In vitro release kinetic studies, as well as pharmacokinetic studies in pigs confirmed a slow liberation of pimecrolimus (40%, 60%, 84% of total drug load being eluted at 1, 4, 12 weeks, respectively). Studies conducted on rabbits showed excellent endothelialisation after two weeks when compared to the first generation commercially available DES.
The complete set of preclinical data was reviewed and validated by independent experts prior to initialising the clinical phase.
Procedure
For this study the pimecrolimus eluting stent was available in two diameters (3.0 and 3.5 mm) and a single length (18 mm). Predilatation was performed in 77% of the cases prior to the stent implantation. Where needed, based on the post-implantation intravascular ultrasound (IVUS), high-pressure post-dilatation was performed.
Angiography was performed at baseline, post-procedure and 6-month follow-up, after intracoronary administration of nitrates. The exact angiographic views performed at baseline were reproduced at follow-up examination. Lesion morphology was assessed using the modified American College of Cardiology/American Heart Association (ACC/AHA) classification system. Quantitative angiographic analysis was performed in the stent segment, (“in-stent”) as well as in the vessel segment (“in-segment”), including the 5 mm proximal and distal to the stent. The quantitative angiographic parameters included: the reference vessel and minimal luminal diameter (MLD); the lesion length; the percent diameter stenosis and the late loss (difference between MLD at the end of the procedure and MLD at follow-up). In-stent and in-segment restenosis were defined as a diameter stenosis > 50% at follow-up located within the stent and the vessel segment, respectively.
IVUS was performed pre- and post-stenting after administration of 100-200 µg of nitroglycerin. A 40 MHZ rotational transducer catheter (Boston Scientific, Natick, MA, USA) with an automatic motorised pullback was utilised. Quantitative parameters of the lumen, stent and vessel cross-sectional area were determined. Neointimal area was calculated as the stent minus the lumen area at follow-up. Lumen, stent, vessel and neointimal volumes were calculated with the QIVUS software (Medis, Leiden, The Netherlands).
Dual antiplatelet therapy including aspirin (at least 75 mg/day with a pre-procedure loading dose of 200-325 mg) and the platelet P2Y receptor antagonist clopidogrel, administered either at 300 mg six hours prior to the procedure, or 600 mg during the procedure, was achieved in all the patients. During the procedure heparin was administered following sheath insertion in order to achieve a recommended activated clotting time > 250 sec. Use of additional medications during the procedure, including GP IIb-IIIa inhibitors was left at the operator’s discretion.
Follow-up
Dual antiplatelet therapy was continued administering acetylsalicylic acid (at least 75 mg/day) indefinitely and clopidogrel (75 mg/day) for six months following the implantation. The first clinical follow-up was performed at 30±7 days after discharge to determine the rate of major adverse cardiac events (MACE). A clinical, angiographic and IVUS follow-up was performed at six months post-procedure for endpoint determination.
Study endpoints
Primary endpoint
The primary endpoint of this trial was a composite of major adverse cardiac events (MACE) at 180±14 days. MACE is defined as cardiac death, myocardial infarction (MI, Q-wave and non-Q-wave) and clinically driven target lesion revascularisation (TLR). The MACE rate was expected to be below 20% to show safety and efficacy within the range of currently available stent systems.
Secondary endpoints
The following secondary endpoints were: in-stent and in-segment binary restenosis rate by angiography (≥ 50% diameter stenosis) at 180±14 days post-procedure; minimal lumen diameter (MLD), late lumen loss (LLL); target lesion revascularisation (TLR), target vessel revascularisation (TVR) at one and six months and at one, two and three years; MACE at one month post-procedure and yearly up to three years; all serious adverse events (SAEs) including all deaths, stent thrombosis at one and six months and at one, two and three years post-procedure; neointimal hyperplasia volume at six months post-procedure measured by IVUS.
Lesion treatment success was defined as the achievement of < 50% residual stenosis by offline QCA using any percutaneous method. Device success was defined as the achievement of a final residual diameter stenosis of < 50% by offline QCA, using the assigned device only.
Procedure success was defined as the achievement of a final diameter stenosis of < 50% measured by offline QCA using any percutaneous method, without the occurrence of death, Q-wave or non Q-wave MI, or repeat revascularisation of the target lesion during the hospitalisation.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation. Frequencies are shown as percentages (%). Comparisons between post-procedural and follow-up parameters were performed using a two tailed paired T test or ANOVA as required. A p value < 0.05 was considered statistically significant.
Results
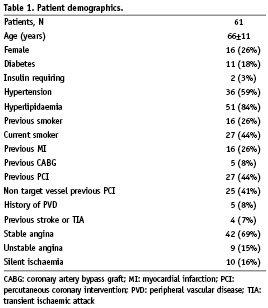
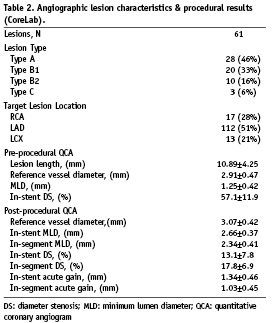
The baseline demographic and the angiographic characteristics of all 61 subjects are displayed in Tables 1 and 2, respectively. The majority of subjects had stable angina (69%) that was mainly CCS Class 2 (41%). Subjects with unstable angina (15%) were mostly classified Braunwald II (10%). The majority of the patients had single vessel disease (59%), and about one-third 2-vessel disease (28%). The culprit lesion was predominantly located in the LAD (51%) and the treated lesions were mostly of type A (46%). Post-dilatation was performed in 64% of the patients with a mean pressure of 17 atm. In-stent residual stenosis was 13% on average and was < 15% in 41 (67%) patients. IVUS detected post-procedural stent malapposition was 14%. Procedural success rate was 100%. All patients were discharged without complications following the procedure.
Sixty patients returned for the 30±7 day follow-up visit at which time MACE rate was 1.6% (Table 3). One patient died six days following implantation of the pimecrolimus-eluting stent because of stent thrombosis and fatal myocardial infarction. Based on the information received from the family members, the patient did not take any of the prescribed anti-platelet medication. This and another case were adjudicated by the Clinical Event Committee as stent thrombosis. The second patient showed a total occlusion at 182 days post-procedure.
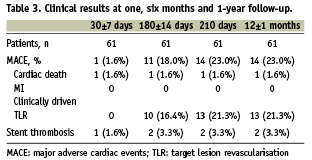
At 6-month clinical follow-up, 71% of the patients were angina free, 27% of the patients presented with stable and 2% with unstable angina. MACE rate at 180 ±14 days was 18.0%. Overall TLR rate was 27.9%. TVR rate was identical to the TLR rate. The number of clinically driven TLR was 16.4% at this time point. Since 8 (13.6%) angiographic controls were done after 194 days, clinically relevant data are shown up to 210 days. MACE rate at 210 days increased to 23% and overall TLR rate was 32.8%. Follow-up at one year showed no additional TLR / TVR (Table 3 and Figure 1). At one year follow-up, there were no cases of a second intervention following the initial repeat intervention that used balloon angioplasty in two, DES stent implantation in 16 and bare metal stent (BMS) implantation in two cases.
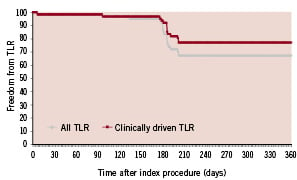
Figure 1. Freedom from overall TLR and clinically-driven TLR.
Quantitative coronary angiography findings are presented in Table 4. Binary in-segment restenosis (restenosis >50%) was observed in 19 subjects (34.5%) and was mostly located within the stent (18 cases, 32.7%). In-stent late lumen loss was 1.11±0.65 mm and in-segment late lumen loss was 1.18±0.59 mm. Cumulative frequency distribution for in-stent diameter stenosis is presented in Figure 2.
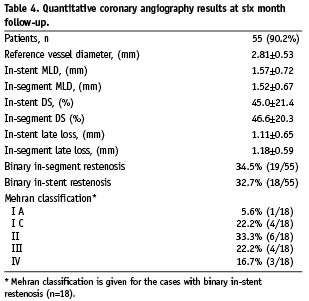
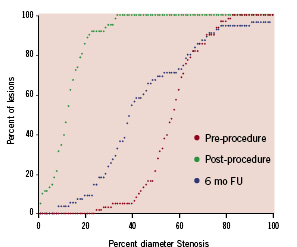
Figure 2. Cumulative frequency distribution for in-stent diameter stenosis pre-, post-procedure and at 6-month follow-up.
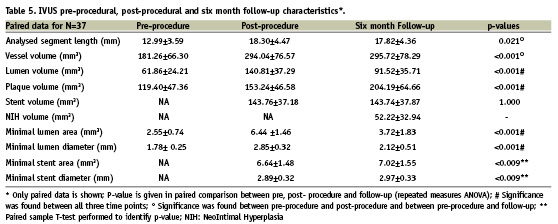
Pre-procedure, post-procedure and follow-up quantitative IVUS measurements of paired data are presented in Table 5. Persistent stent malapposition was detected in 8.5% of cases at follow-up and no late acquired malapposition was observed. In-growth of neointimal hyperplasia volume was 52.22±32.94 mm.
Discussion
This study is the first-in-man, non-randomised, multicentre trial with the pimecrolimus eluting stent. Although the observed MACE rate was within the boundaries of the predefined endpoint at 180±14 days (MACE rate occurrence < 20%), this new device did not reach the expected results in terms of anti-restenotic efficacy.
The three components of the pimecrolimus eluting stent are: the CoCr metal stent coated with an intermediate layer of amorphous hydrogen-rich silicon carbide (aSiC:H), the bioresorbable poly-L-lactic acid polymer and pimecrolimus. The combination of these components was expected to show advantages in reducing inflammatory activity, neo-intimal proliferation and preventing delayed re-endothelialisation. The pimecrolimus drug represented the true innovation of this stent system due to its broader therapeutic window and reduced vascular toxicity. The structure of pimecrolimus belongs to the macrolactam group of agents and it is similar to the tacrolimus structure, but the higher lipophilicity of pimecrolimus allows a longer permanence in the tissues that can use it as reservoir. The preclinical data derived from more than 160 pimecrolimus eluting stents implanted into different species did proof safety and drug efficacy in vivo.
However, the clinical experience did not match the pre-clinical expectations. The in-stent late lumen loss of 1.11 mm is higher than that of currently approved drug eluting stents - ENDEAVOR™ (~0.6 mm), TAXUS™ (~0.4 mm), CYPHER™ (~0.15 mm), XIENCE-V™ (0.1 mm). MACE rate (23%), in-stent binary restenosis rate (33%) and in-segment binary restenosis rate (34.5%) up to 210 days were high compared to currently available DES. Similar clinical results to this study have been published with other DESs using pimecrolimus. The first-in-man study of a stainless steel stent coated with a durable pimecrolimus eluting polymer (Avantec Vascular Corp., Sunnyvale, CA, USA) reported an in-stent late loss of 1.44 mm ± 0.89 at six months follow-up. The Corio™ stent (Conor Medsystems, Menlo Park, CA, USA) which was a cobalt chromium stent, eluting pimecrolimus from reservoirs with a poly lactide-co-glycolide resorbable polymer, had an in-stent late loss 1.40 ± 0.67 mm, and a MACE rate of 39% at six months in the GENESIS trial. The clinical results for all three pimecrolimus eluting stents are comparable with the drug being the common component of all three drug/polymer/stent systems.
In contrast to many other DES systems, there seems to be a mismatch between preclinical and clinical data obtained with pimecrolimus drug-eluting stents. Pharmacological studies are needed to investigate the reasons for these species dependent differences. In any case, the current evidence from this and the two other clinical trials have shown that pimecrolimus lacks efficacy, and this currently disqualifies it from being used in DES applications in humans.
Limitations of the study
The lack of a control group results in the limitation that a direct comparison with a different BMS/DES is not possible. The results can only be compared to a historical control group.
Conclusions
The anticipated anti-restenotic effect of the ProGenic Stent did not meet expectations. The use of pimecrolimus as an anti-inflammatory agent does not appear to be a viable option for future DES development. Of note, in-stent restenosis responded favourably to a single re-intervention with good clinical outcome up to one year.
Acknowledgements
The outstanding contributions of Anita Patteet, Sandra Hujer and Michael Tittelbach are gratefully acknowledged.

