
Abstract
Aims: In preclinical studies, a bare metal cobalt-chromium stent with an active surface oxide layer modification (BMSmod) has been shown to inhibit neointimal hyperplasia effectively. We sought to assess both the clinical safety and feasibility of the BMSmod.
Methods and results: In this prospective, non-randomised, first-in-man multicentre study, a total of 31 patients with de novo coronary lesions, reference lumen diameters of 2.5-3.5 mm and lesion length ≤16 mm, were enrolled. Quantitative coronary angiography and optical coherence tomography (OCT) were performed at baseline and six-month follow-up. Primary angiographic and OCT endpoints included in-stent late lumen loss (LLL) and mean neointimal thickness at six months. The device-oriented composite endpoint (DoCE), defined as cardiac death, myocardial infarction not clearly attributable to a non-intervention vessel, and clinically indicated target lesion revascularisation (CI-TLR), was analysed according to the intention-to-treat principle. In 31 patients (33 lesions), the procedural success rate was 93.5%. At six months, angiographic LLL was 0.91±0.45 mm and binary angiographic restenosis occurred in 23.3% of lesions. Out of 33 lesions, OCT was performed in 27 lesions at both time points. Mean neointimal thickness amounted to 348±116 µm. At six months, the DoCE was 19.4% due to the occurrence of CI-TLR in five patients (including one late definite stent thrombosis of a non-study stent).
Conclusions: In contrast to previous preclinical pathophysiological work, the BMSmod did not prevent neointimal hyperplasia in a first-in-man clinical setting. Clinical Trials registration: NCT02176265
Abbreviations
BMS: bare metal stent
CI-TLR: clinically indicated target lesion revascularisation
DS: diameter stenosis
ISA: incomplete strut apposition
MLD: minimal lumen diameter
MLA: minimal lumen area
OCT: optical coherence tomography
QCA: quantitative coronary angiography
RLD: reference lumen diameter
Introduction
Despite significant therapeutic successes achieved by the first-generation drug-eluting stent (DES) in the reduction of restenosis and target vessel revascularisation compared with the bare metal stent (BMS), it has been shown that the first-generation DES was associated with an increase of very late stent thrombosis1 and led to mandatory long-term dual antiplatelet therapy (DAPT)2. With regard to this concern, the second-generation DES was developed and has shown clinical advantage over the first-generation DES, resulting in a six-month duration of DAPT3. Nevertheless, the new BMS platform, with new designs, metal composition, thinner struts and surface modification has also been developed in parallel with the second-generation DES to inhibit neointimal hyperplasia actively and reduce clinical adverse event rates with short-term DAPT.
The Qvanteq surface-modified coronary stent system is a cobalt-chromium (CoCr) BMS which has undergone oxide surface layer modification (BMSmod; Qvanteq AG, Zurich, Switzerland). The modification reduces nickel and cobalt concentrations in the surface oxide and simultaneously removes organic contamination, resulting in an ultra-hydrophilic surface that facilitates fast endothelial growth4. It has been hypothesised that surface treatment may offer a safe and effective alternative to DES with rapid healing and short duration of DAPT. In a porcine model with healthy coronary arteries, the BMSmod demonstrated effective inhibition of neointimal formation5.
The present first-in-man (FIM) study was designed to test the safety and feasibility of the BMSmod for the treatment of de novo coronary lesions.
Methods
PATIENT POPULATION
The study enrolled 31 patients at six participating sites in Switzerland and the Netherlands. A total of 31 patients over 18 years of age with stable or unstable angina (with a stable haemodynamic condition) or silent ischaemia with a single de novo target lesion of >50% diameter stenosis and <16 mm with a diameter 2.5-3.5 mm in one or two major epicardial arteries were included. Key exclusion criteria were: 1) evidence of ongoing acute myocardial infarction (MI) prior to the procedure; 2) stroke/transient ischaemic attack in the past six months; 3) left ventricular ejection fraction <30%; 4) known hypersensitivity or contraindication to aspirin, heparin, clopidogrel or cobalt-chromium; 5) requirement for oral anticoagulation or prolonged need for DAPT; 5) other medical illness that may cause non-compliance with the protocol; 6) female of child-bearing potential; and 7) recipient of a heart transplant. Angiographic exclusion criteria included: severe tortuous, calcified or angulated coronary anatomy of the study vessel; target lesion in left main stem; involving a side branch >2.0 mm in diameter; aorto-ostial lesion; total occlusion; visible thrombus; restenotic lesion; and arterial or saphenous vein graft lesions.
The study protocol was approved by all local institutional ethics committees and informed consent was obtained for every patient before any intervention was performed.
DEVICE DESCRIPTION
The BMSmod is a CoCr stent which has undergone surface treatment by modifying the native oxide layer composition, as described elsewhere4. In vitro studies have shown that the treated surface reduces platelet adhesion while increasing the adhesion of polymorphonuclear neutrophils4. The increase of neutrophils leads to the neutrophil-released protein “cathelicidin” which reduces neointimal formation6. Therefore, the BMSmod is expected to have less thrombogenicity and in-stent restenosis4,7. The BMSmod is a balloon-expandable stent compatible with a 6 Fr guide catheter. The stent is arranged inside a container in an inert environment to protect its hydrophilic surface properties. It was mandatory to fill the guiding catheter with blood by back bleeding so that the stent’s surface would first be in direct contact with blood. The balloon delivery system has two radiopaque markers to aid in the placement of the stent during fluoroscopy. The strut thickness is 80 µm for the diameter 2.75 mm and 90 µm for the diameter 3.00 mm.
STUDY PROCEDURE
Lesions were treated using standard interventional techniques with mandatory predilatation prior to stent implantation. The following sizes of BMSmod were used in the study: 15 mm and 20 mm length and either 2.75 or 3.00 mm diameter. The treating physicians performed the procedure using only angiographic guidance. OCT was performed after angiographic optimal stent placement and the physicians performing the procedure were blinded with respect to OCT images and results. Preprocedural antiplatelet therapy followed current guidelines8. Post procedure, patients were required to take clopidogrel 75 mg once daily for one month and aspirin 75-100 mg once daily indefinitely.
Angiographic and OCT evaluations were performed post procedure and at six months. An independent data safety monitoring board monitored the individual and collective safety of the patients in the study on an ongoing basis.
QUANTITATIVE CORONARY ANALYSIS
Two-dimensional quantitative coronary analysis (QCA) was performed by an independent core lab (Cardialysis BV, Rotterdam, the Netherlands) with the CAAS system (CAAS 5.11; Pie Medical Imaging BV, Maastricht, the Netherlands).
OCT ACQUISITION AND ANALYSIS
OCT was performed using three different frequency-domain OCT systems according to the availability at the participating sites (OPTIS™ Integrated System; ILUMIEN™ OPTIS™ PCI Optimization™ System and Dragonfly™ Duo Imaging Catheter; C7-XR™ OCT Intravascular Imaging System and Dragonfly™ Catheter; all St. Jude Medical, St. Paul, MN, USA). All OCT images were analysed by an independent core laboratory (Cardialysis BV) with QIvus 2.2 software (Medis, Leiden, the Netherlands). Cross-sectional OCT images were analysed at 1 mm intervals.
OCT DEFINITION
A covered strut was defined as having a neointimal thickness more than 0 µm9. Incomplete strut apposition (ISA) was defined as a clear separation between strut and vessel wall with a distance greater than the thickness of the strut. The healing score (HS)10 was calculated at every time point as previously described in detail elsewhere. The stent expansion index was calculated by the ratio of minimum stent area (MSA) to the average reference lumen area (RLA). The optimal stent expansion (OSE) was defined according to the criteria of the MUSIC study11.
EVALUATION OF DEGREE OF STRUT EMBEDMENT AND NEOINTIMAL RESPONSE
An exploratory analysis of strut embedment was performed using dedicated software (LKEB, Leiden University, the Netherlands) for quantification of the degree of strut embedment. The algorithm and reproducibility of the embedment software have been described elsewhere12. Briefly, the centre of the reflective border of the metallic strut is detected automatically by the software. The abluminal side of the metallic struts is automatically drawn by simulating the virtual contour of the struts using the thickness of the strut indicated by the manufacturer. The degree of strut embedment is reported as “embedment depth”. Embedment depth is the distance between the abluminal side and the embedment line measured along the line from the abluminal side through the lumen centre. An example of the measurement of stent strut embedment is illustrated in Figure 1.
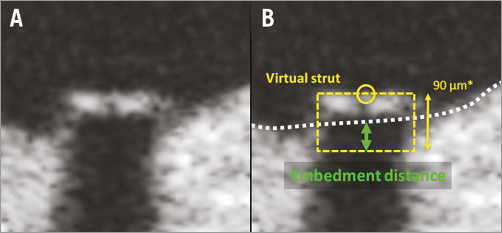
Figure 1. Method of the OCT embedment analysis. A strut example is illustrated without contour (A) and with contour (B). The dedicated software automatically creates the virtual struts (yellow dotted square) based on the strut thickness (BMSmod 2.75 mm device=80 μm; and *3.0 mm device=90 μm) provided by the manufacturer. Embedment depth (green arrow) was computed using the interpolated lumen contour (white dotted line).
STUDY ENDPOINTS
The primary angiographic endpoint was six-month in-stent late lumen loss (LLL). The primary OCT endpoint was six-month mean neointimal thickness. The secondary clinical endpoints included device-oriented composite endpoints (DoCE) (defined as cardiac death, MI not clearly attributable to a non-intervention vessel, and clinically indicated target lesion revascularisation [CI-TLR])13 and the individual components of the composite endpoint, any revascularisation at six and 12 months and stent thrombosis according to the Academic Research Consortium (ARC) definitions13 up to 12-month follow-up. Periprocedural MI and spontaneous MI were defined by the third universal definition and WHO definition. Device success was defined as DS <30% of the target site using the BMSmod. The procedure success required DS <30%, and no occurrence of DoCE during the hospital stay. All patients visited the outpatient clinic at six months. Telephone contacts were scheduled at 12 months.
STATISTICAL ANALYSIS
Categorical variables were summarised with frequencies and percentages. The continuous variables were reported as mean±standard deviation (SD) or median and interquartile ranges as appropriate. The Wilcoxon signed-rank test was used to compare continuous variables between serial OCT and QCA data. The categorical variables were compared by Fisher’s exact test. A two-sided p-value <0.05 was considered statistically significant. The current study is a FIM and single-arm study; the sample size was not defined on the basis of an endpoint hypothesis but rather to provide some information about the device safety. The primary endpoint and all imaging-based findings were analysed based on the as-treated population. Clinical outcome analyses were based on the intention-to-treat population.
The comparison between neointimal response (area, thickness, volume) and embedment depth was performed by dividing the analysed cross-sections into three sub-segments as: proximal, mid, and distal. The correlation between embedment depth and neointimal component was compared by using sub-segment level. The statistical analysis was performed using SPSS, Version 23.0 (IBM Corp., Armonk, NY, USA).
Results
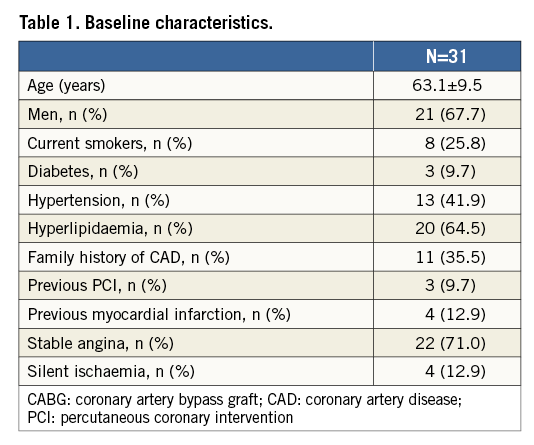
BASELINE CLINICAL CHARACTERISTICS
Thirty-one patients were included between October 2014 and August 2015. The baseline clinical characteristics are presented in Table 1. Procedure success was 93.5% (29/31) and device success was 93.9% (31/33) of the lesions because two study stents could not cross the lesions. There were three study stent dislodgements due to the larger profile of the study device (6 Fr guide catheter compatibility) as compared to lower profile devices (5 Fr guide catheter compatibility) currently used in clinical practice: the first stent got dislodged from the balloon during the use of a “mother-and-child” configuration (5 Fr). A non-study stent (Promus PREMIER™; Boston Scientific, Marlborough, MA, USA) was subsequently implanted. In the second case, the stent dislodgement occurred in the Y-connector outside the patient when the investigator attempted to implant a study stent in the mid RCA. A non-study stent (XIENCE PRIME™; Abbott Vascular, Santa Clara, CA, USA) was implanted. The last stent dislodgement occurred because the mandatory predilatation of the lesion was omitted and the study stent device profile (1.6 mm) was too large for crossing the lesion (MLD 0.84 mm). The stent got dislodged while the operator was retrieving it back into the guide catheter. Subsequently, predilatation was performed and another study stent was successfully implanted in the index lesion.
ANGIOGRAPHIC AND PROCEDURAL CHARACTERISTICS
Baseline angiographic characteristics are shown in Table 2. Angiographic follow-up at six months was achieved in 28 patients (30 lesions) (Figure 2). Serial angiographic analyses at baseline, post procedure, and six-month follow-up (n=30 lesions) are presented in Table 3. At six months, the mean in-stent late loss, in-stent percentage diameter stenosis, and the frequency of binary angiographic restenosis were 0.91±0.45 mm, 36.0±18.1%, and 23.3%, respectively. Figure 3 demonstrates the cumulative frequency of MLDs immediately after the index procedure and at six-month follow-up, and in-stent LLL.
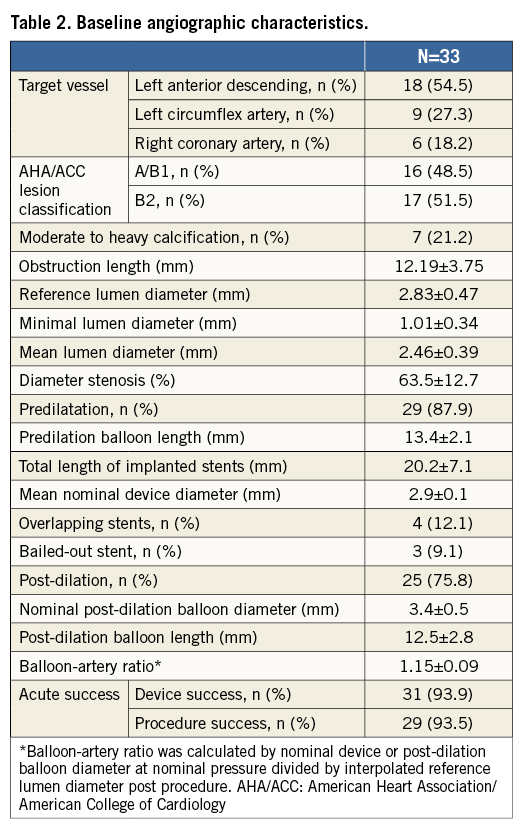
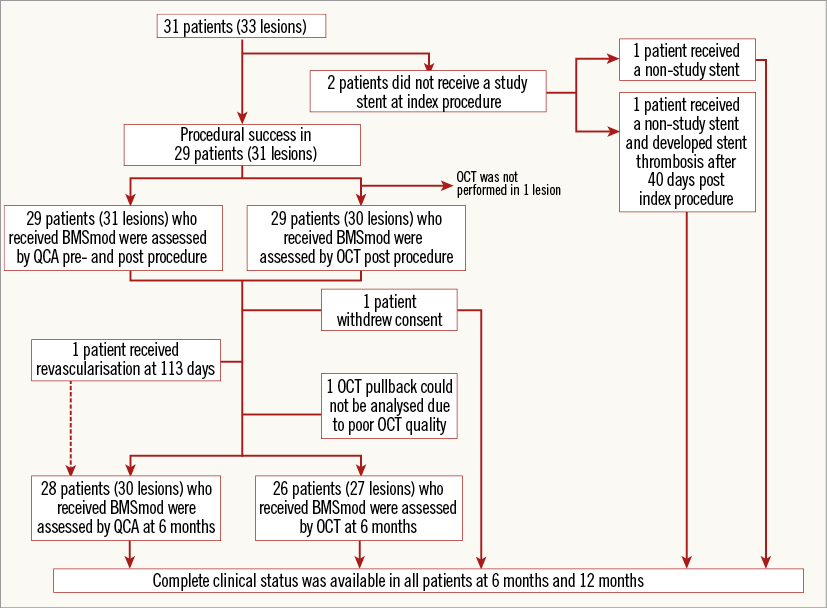
Figure 2. Study flow chart.
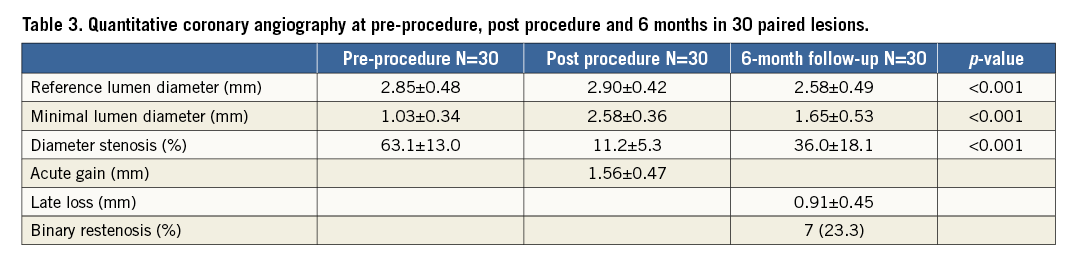
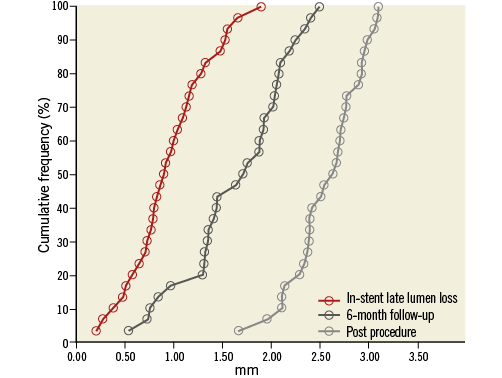
Figure 3. Cumulative frequency distribution curves of minimal luminal diameter at post procedure (pale grey line) and at six-month follow-up (dark grey line), and of in-stent late lumen loss at six months (red line).
OCT FINDINGS AT BASELINE AND AT SIX MONTHS
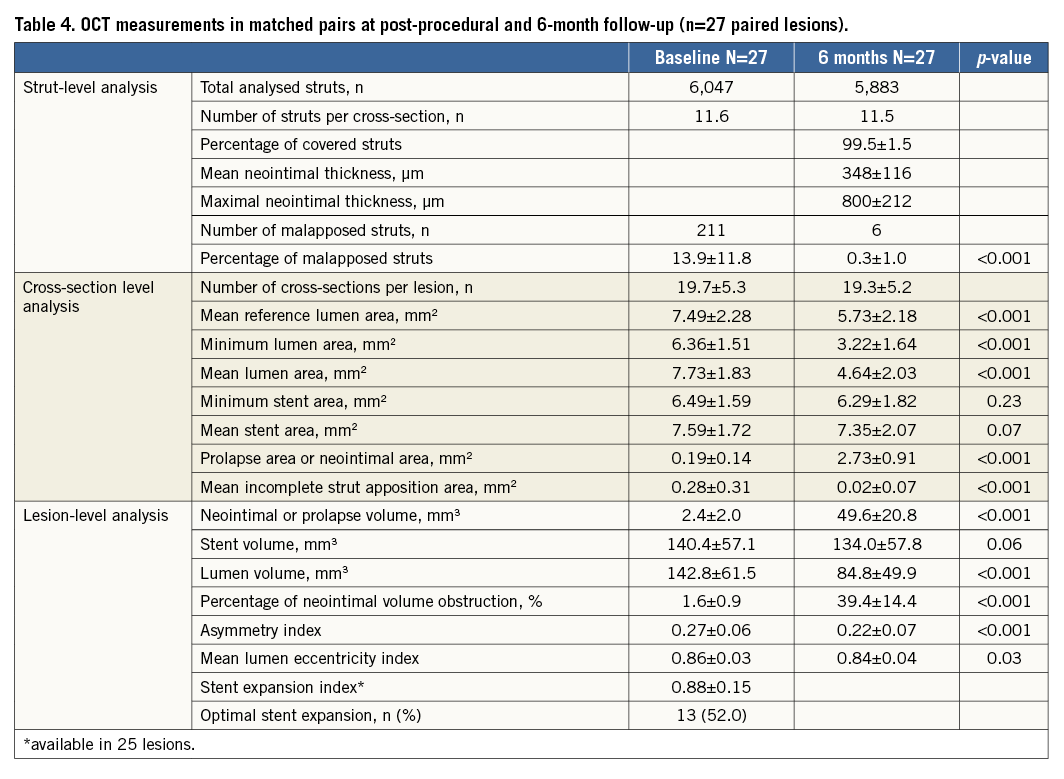
At six months, OCT evaluation was performed in 26 patients (27 lesions). The results of paired OCT at baseline and six-month follow-up are tabulated in Table 4. The six-month mean neointimal thickness was 348±116 µm with % NVO of 39.4±14.4%. The percentage of covered struts was 99.5±1.5% with a healing score of 4.3±13.9. ISA at baseline was reported in 13.9±11.8% of the analysed struts, and that percentage was reduced to 0.3±1.0% at six months. There was no late acquired incomplete apposition.
CORRELATION OF NEOINTIMAL RESPONSE TO THE EMBEDMENT DEPTH
The mean strut embedment depth was 70.4±28.4 µm after stent implantation. The embedment depth was deepest in the middle segment, followed by the proximal and distal segments, respectively (Table 5). The neointimal thickness, neointimal area and neointimal volume were also numerically highest in the middle segment; however, there were no statistically significant differences between the proximal and distal segment. There was a moderate positive correlation between neointimal area, thickness and neointimal volume and embedment depth: r=0.320, p=0.005, r=0.424, p<0.001, and r=0.374, p=0.001, respectively (Figure 4, Figure 5).
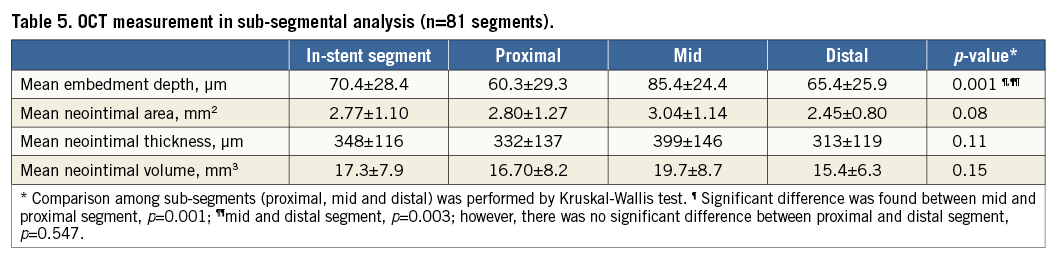
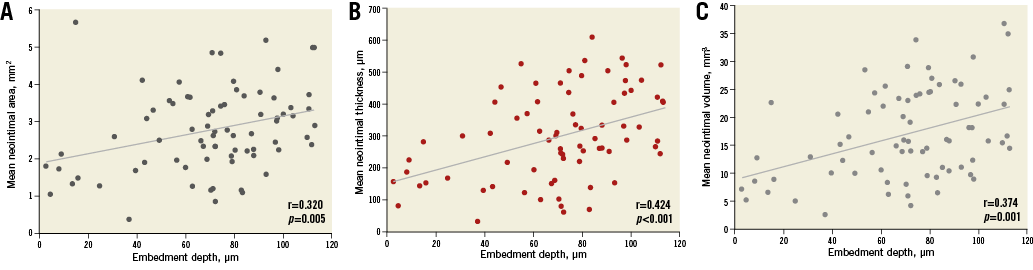
Figure 4. Correlation between the degree of strut embedment and neointimal response. Strut embedment to mean neointimal area (A), neointimal thickness (B), and neointimal volume (C).
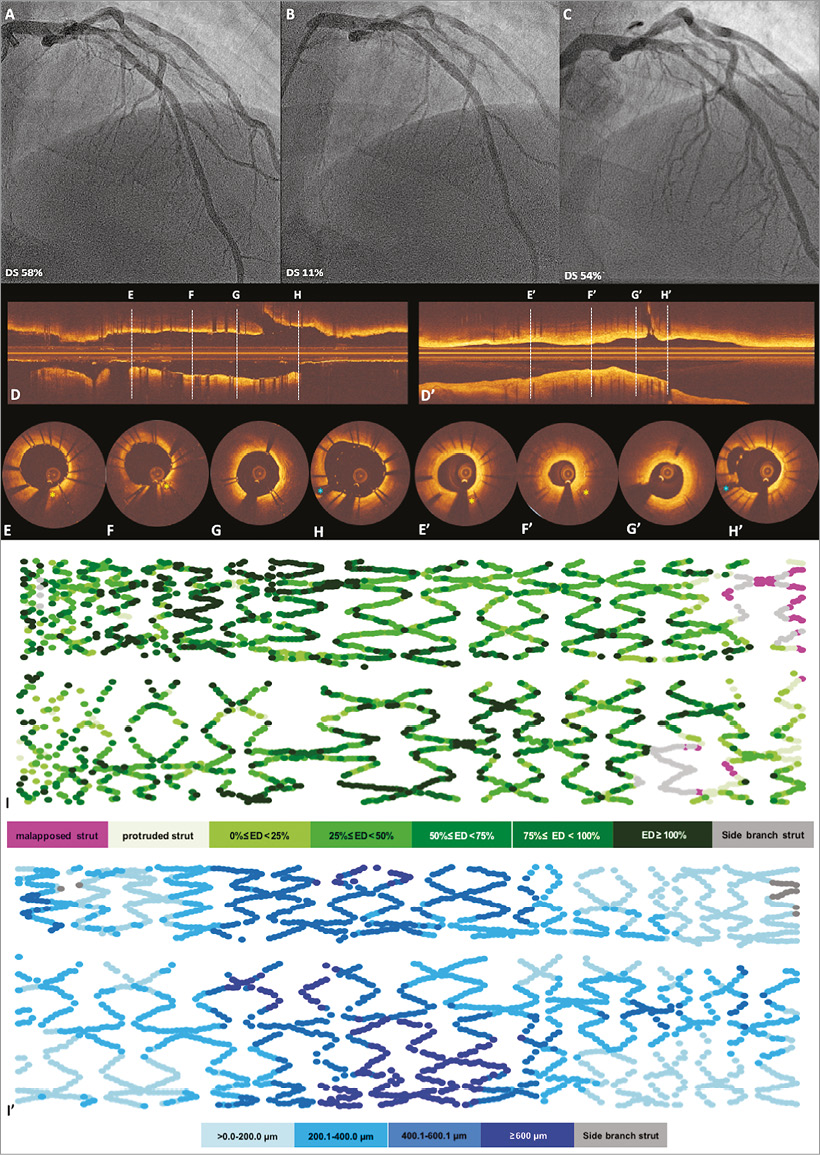
Figure 5. Case examples of embedment analysis at baseline and neointimal thickness at six-month follow-up. A patient received BMSmod 3.0×20 mm in the proximal left anterior ascending artery. A, B and C demonstrate angiography pre-procedure, post procedure and at six months, respectively. The six-month angiographic late loss was 1.28 mm. Optical coherence tomographic (OCT) imaging (longitudinal view: D, cross-sections: E-H) showed embedded (E-G), buried (F) and malapposed struts (H) at baseline. The yellow stars indicate calcific plaque, and the blue star indicates the side branch, both of them used as anatomical landmarks for matching OCT images between baseline and six-month follow-up. The corresponding longitudinal view and cross-section at six-month follow-up are shown in D’ and E’- H’, respectively. Panel F’ shows that minimal lumen area at six-month follow-up was 2.48 mm2. The OCT foldout view in panel I depicts the distribution of malapposed struts and embedment depth of each strut, colour coded in lilac and green, respectively. The healing score of the case at baseline was 234.9 and decreased to 11.54 at six months. The OCT foldout view in panel I’ depicts the distribution of the neointimal thickness of each strut, colour coded in blue shades.
DEVICE-ORIENTED COMPOSITE ENDPOINTS (DoCE)
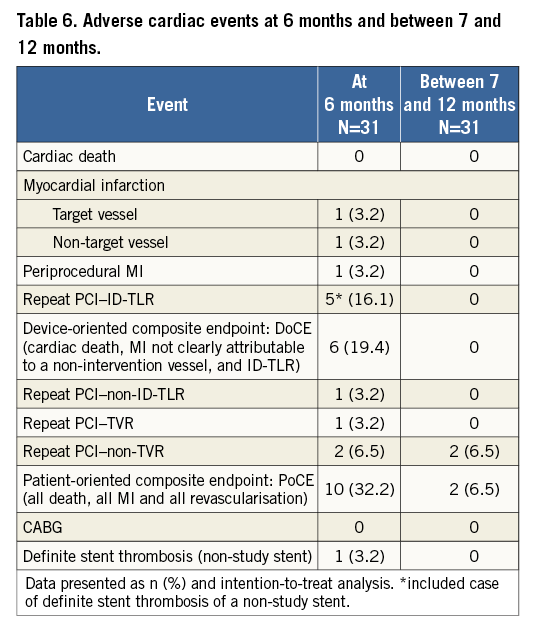
Clinical data were available up to 12 months in all patients. Individual clinical endpoints are listed in Table 6. One periprocedural MI occurred (cTn 29.57xULN, CK-MB 6.43xULN with symptom). One patient experienced a definite late stent thrombosis of a non-study stent at 40 days after the index procedure. There were five cases of CI-TLR (including one case with definite stent thrombosis in a non-study stent). In total, the DoCE rate at six months was 19.4% (6/31). Including only patients in whom the device was implanted successfully, the DoCE rate at six months was 17.2% (5/29). No additional DoCE occurred between seven months and 12 months.
Discussion
The main findings of this first-in-man study are the following: 1) while the surface technology itself appeared to be safe in the context of the present study, the BMSmod was associated with in-stent angiographic late loss of 0.91 mm and a six-month binary restenosis rate of 23.3%; 2) the endothelialisation of the BMSmod was almost complete at six months (percent covered struts 99.5%); 3) the six-month mean neointimal thickness was 348±116 µm with a % NVO of 39.4±14.4%.
The present study showed that the neointimal hyperplasia in the BMSmod was not substantially reduced as compared to the expected LLL in currently used BMS14 (BMSmod 0.91±0.45 mm, MULTI-LINK VISION® [Abbott Vascular] 0.87±0.37 mm). The same observation was made in the OCT analysis: % NVO of the BMSmod was comparable to the MULTI-LINK VISION (BMSmod 39.4±14.4% vs. MULTI-LINK VISION 28.1±14.0%)14.
THE IMPACT OF SURFACE MODIFICATION ON THE NEOINTIMAL RESPONSE
Recently, the BMSmod showed promising results in inhibiting neointimal proliferation in rabbit and swine models compared to untreated surface BMS and DES5, whereas BMSmod efficacy in the current FIM study showed results comparable with current-generation BMS.
Although the balloon-to-artery ratio was comparable between the previous animal models and the present FIM trial (1.14±0.05 vs. 1.15±0.09), the balloon-to-artery ratio at the MLA site would be greater than at any other area in the vessel. Consequently, a huge stretch at the MLA site by the balloon could contribute to severe injury to the vessel wall. On the other hand, the balloon-to-artery ratio in the animal model was homogeneous throughout the stent length since there was no pathological stenosis. Therefore, the antirestenotic property of the BMSmod could not be adequately tested at 30 days in the porcine model.
VESSEL INJURY AFTER IMPLANTATION AND NEOINTIMAL RESPONSE
Previously, Schwartz et al reported that the mean histologic injury score significantly correlated with histologic neointimal thickness, percent area stenosis post procedure and neointimal area (r=0.80, p<0.001; r=0.74, p<0.001, and r=0.46, p=0.02, respectively)15. However, the correlation of the neointimal response and the embedment depth in the present study was not as strong as in the histological assessment. Part of the discrepancy might be explained by the different properties of diseased human coronary artery tissue as compared to healthy porcine models16 and the limitation of OCT resolution to detect media disruption.
TRANSLATION OF THE PRECLINICAL STUDY TO THE FIRST-IN-MAN TRIAL
Theoretically, the objectives of a FIM stent trial are to ascertain safety and feasibility but not mandatorily to address the question of the efficacy of the device. There is a heavy ethical responsibility involved in FIM trials. In converting the preclinical result into therapeutic applications, it has been our experience that stent treatment in the healthy coronary arteries of juvenile growing animals (mainly pigs) is frequently misleading and tests only the compatibility of the device with the biological environment without testing per se the antirestenotic property of the device17,18. In order to test the antirestenotic properties, a certain level of injury should be induced and quantified by imaging to establish the relationship observed between vessel injury and neointimal hyperplasia15. Additionally, this type of preclinical work should be performed in mature animals that are not going to grow excessively at follow-up (e.g., mini Yucatan swine) and the investigation should induce a quantified level of device injury17,18.
Study limitations
The following limitations should be acknowledged. 1) Based on the characteristics of this FIM study, the study population was not large enough to address fully the question of efficacy. 2) Based on an interim analysis and consideration of the three stent dislodgements, recruitment was stopped after 31 patients, instead of after the planned 35 patients. The procedural success rate of the device in its current version was suboptimal, warranting further refinement. Device dislodgements may be explained by the 6 Fr compatibility profile of the study device that was larger than most stent platforms currently used in clinical practice, together with a wrong device handling as described above. Thus, development of novel platforms should target inhibition of neointimal hyperplasia and the profile of the device on the delivery system should also be taken into account for deliverability, pushability and retention. 3) The OSE criteria were derived from IVUS criteria in the MUSIC study, although OCT was used in the current study. A reported discrepancy between OCT and IVUS measurements could influence the assessment of OSE criteria19. However, the influence seems to be minimal since the stent expansion was assessed as a ratio of MSA to RLA, not as absolute values.
Conclusions
Despite the conceptual advantages of surface modification and effective inhibition of neointimal growth in animal models, this FIM study showed that the biocompatibility-focused surface modification is not sufficient to reduce the neointimal growth resulting from the overstretching dilatation of human coronary atherosclerotic narrowing. However, the observed efficacy was comparable with current-generation BMS. Preclinical studies with new stent platforms should induce a certain level of injury that would mimic the barotrauma in human atherosclerotic stenosis.
| Impact on daily practice Surface modification technology has demonstrated an effective inhibition of neointimal growth and rapid healing in preclinical studies. It has been speculated that such technology may offer alternatives to DES when short dual antiplatelet therapy is mandatory. This FIM study showed that the surface modification is not sufficient to reduce the neointimal growth in diseased human coronary arteries. Considering the recently well proven efficacy and safety of the drug-coated stents versus BMS in high bleeding risk patients, DES will remain in our armamentarium in the future unless new BMS platforms demonstrate a comparable antirestenotic capability to DES. In converting the future preclinical results of any new BMS platforms into therapeutic applications, such devices should be tested for biocompatibility and antirestenotic properties by induction of a quantified level of device injury. |
Guest Editor
This paper was guest edited by Fernando Alfonso, MD, PhD, FESC; Servicio de Cardiología, Hospital Universitario de La Princesa, Universidad Autónoma de Madrid, Madrid, Spain.
Conflict of interest statement
L. Räber and J. Daemen are principal investigators of the trial. P.W. Serruys is chairman of the trial. Y. Onuma is the director of the core lab at Cardialysis BV. C. von Birgelen reports institutional research grants from AstraZeneca, Biotronik, Boston Scientific, and Medtronic. S. Buzzi and A. Zucker are employees of the trial’s sponsor. S. Windecker reports institutional research grants from Abbott, Biotronik, Boston Scientific, Edwards, Medtronic and St. Jude. The other authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.

