Abstract
Aims: Revascularisation with Titanium-Nitride-Oxide (TiNOX) coated stents is safe and effective in patients with de novo native coronary artery lesions. In the TiNOX trial there was a reduction in restenosis and major adverse cardiac events as compared with stainless steel stents of otherwise identical design. The purpose of the present study was to evaluate the long-term outcome of these patients over five years.
Methods and results: In 2003, 92 patients with de novo lesions were randomly assigned to treatment with TiNOX coated stents (n=45) or stainless steel stents (n=47; control). Baseline characteristics were similar in both groups. Follow-up at six months and five years was obtained in 87 patients. Five patients were lost to follow-up due to emigration or change in home address. At six months and five years, significantly less major adverse cardiac events (MACE) occurred in the TiNOX group (7% vs. 27%, [p=0.02] respectively and 16% vs. 39%, [p=0.03]), largely driven by a reduced need for target-lesion revascularisation. No stent thrombosis occurred in the TiNOX group vs. one in the control group. Patients in the TiNOX-group had lower all-cause mortality and less myocardial infarction, but the difference was not statistically significant.
Conclusions: Five-year follow-up after implantation of titanium-nitride-oxide coated stents is favourable with a low rate of MACE and no stent thrombosis compared to bare metal stents of identical design. The need for revascularisation at five years was 9% in the TiNOX and 25% in the control group with little progression of native coronary disease.
Abbreviations
MACE: major adverse cardiac events
TiNOX: titanium-nitride-oxide
Introduction
Drug-eluting stents (DES) have been used with great success in the past 5-6 years, with significant reduction in restenosis and need for repeat revascularisation1-5. However, long-term results are sparse and suggest an increased risk of stent thrombosis, a clinical condition associated with a high morbidity and mortality6-8. Several new technologies have been developed in recent years including the use of biodegradable polymers, new drugs within the “-limus” family (everolimus, zotarolimus, biolimus) or extended drug release over several weeks and months9,10 in order to overcome these drawbacks of DES.
A completely different approach has been chosen by using a passive coating of bare metal stents with titanium-nitride-oxide with the aim to: (1) increase corrosion-resistance, (2) to enhance biocompatibility and (3) to improve vascular healing11,12. The short-term results of the TiNOX trial12 showed favourable results with regard to MACE at six months. Binary restenosis was reduced from 33% in the control to 15% in the titanium-nitride oxide coated stent group (p=0.07). These numbers may appear high, but at the time of the study stents had to be crimped manually, which is associated with an increase in restenosis and MACE rate13.
Similarly, data from randomised trials comparing titanium-coated with paclitaxel-eluting stents14 showed similar clinical outcome at 12-month follow-up. The purpose of the present study was to assess the long term outcome of the TiNOX trial population.
Patients and methods
The initial patient population was followed for a total of five years. As described previously (The TiNOX trial12), the original study population (Table 1) consisted of 92 patients with stable or unstable angina pectoris or signs of myocardial ischaemia and de novo lesions in native coronary arteries with a lesion length <15 mm, a reference vessel diameter of 2.5 to 3.5 mm, and a diameter stenosis of ≥50%.
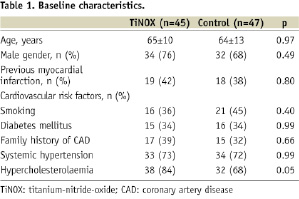
No more than two target lesions were treated in a single patient, and the second lesion had to be located in another major epicardial coronary artery. Exclusion criteria included acute myocardial infarction; severe heart failure; cardiogenic shock; restenotic lesions; a target lesion of the left main or in a vessel with thrombus, or severe calcification; severe comorbidities with a life expectancy of <1 year; lack of informed consent; or unwillingness to undergo coronary angiography during follow-up. The study was conducted according to the declaration of Helsinki, and was approved by the institutional review boards of all participating institutions. Written informed consent was obtained from all patients. The study was a prospective, single-blind, randomised, multicentre trial performed in five centres, three in Switzerland and two in Germany. Patients were randomly assigned to receive either a titanium-nitride-oxide coated or an uncoated, stainless steel stent of otherwise identical design. Randomisation was performed by means of sealed envelopes supplied to each participating centre from the study coordinating centre. The stents were visually distinguishable through the dark-surface appearance of the titanium-nitride-oxide coated stents, which left the patient – but not the implanting physician – unaware of which stent was implanted.
Stent coating with titanium-nitride-oxide
A commercially available stainless steel stent with a tubular slotted design (OMEGA, Qualimed Inc, Munich, Germany) was used in the present study. Unmodified stainless steel stents served as controls. Coating of stainless steel stents was performed by physical vapour deposition of titanium in a prespecified gas mixture of nitrogen and oxygen in a vacuum chamber as previously described12.
Stent implantation procedure
All patients were pretreated with aspirin (100 mg daily) and received intravenous heparin (100 IU/kg) during the procedure. Oral clopidogrel was administered as a loading dose of 300 mg before or immediately after the procedure and was continued at a daily dose of 75 mg for one month. The use of glycoprotein IIb/IIIa antagonists was left to the discretion of the operator. All lesions were predilatated with a target balloon-to-artery ratio of 1:1. The stent was then manually crimped on the deflated balloon. After positioning of the stent in the lesion it was implanted with a pressure ranging between 8-12 bar; more than one stent could be implanted into the lesion in case of incomplete lesion coverage, edge dissection, or otherwise suboptimal result12.
Quantitative coronary angiography
Coronary angiograms were analysed by the angiographic core laboratory at the German Heart Centre, Technical University, Munich, Germany. Angiograms were viewed offline with the automated edge-detection system CMS (Medis Medical Imaging Systems, Leiden, The Netherlands). The exact procedure has been described previously (TiNOX trial12). The analysis was performed after the intervention (baseline) and at 6-month follow-up.
Long term follow-up
An attempt was made by the five participating centres to contact all patients at five years of follow-up. If the patient could not be reached, the referring physician was contacted and interviewed on the current status of the patient. The phone interview was structured with questions for well being, current complications, study medication or any adverse event associated with the stent implantation.
Study endpoints and analysis
The primary endpoint of the present study was major adverse cardiac events (MACE) at six months and five years; secondary endpoints were target lesion revascularisation (TLR), all cause mortality, non-fatal or fatal myocardial infarction and stent thrombosis, diagnosed in the presence of an acute coronary syndrome with angiographic evidence of either vessel occlusion or thrombus within the study stent.
Statistical analysis
Results are shown as mean ±SD or as proportions (%). Differences between groups were compared with an unpaired t-test for continuous variables and Fisher’s exact test for categorical variables. Time to event curves were analysed using the Kaplan-Meier method and distributions were compared by the log-rank test. Peto and Peto’s generalised Wilcoxon test and the log-rank test algorithms were used for calculation. P values ≤0.05 were considered significant. All data were analysed with the use of SPSS version 11 (Statsoft Inc, Tulsa, OK, USA).
Results
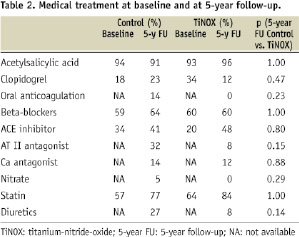
Eighty-seven of the 92 patients could be contacted by phone or through the referring physician. Five patients were lost to follow-up due to emigration out of the country. There was no statistically significant difference regarding medical treatment between the two groups (Table 2).
Clinical outcome
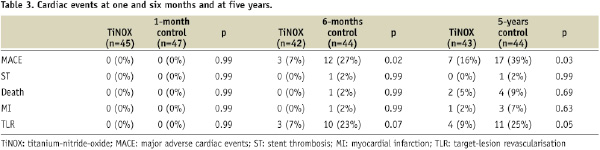
The rate of MACE at six months was 7% in the TiNOX and 27% in the control group12. The difference in MACE was largely due to the higher need for revascularisation (TLR) in the control group (23%) as compared to TiNOX group (7%). Stent thrombosis was rare in the first six months, and occurred only in one patient of the control group.
The rate of MACE at five years increased in both groups from 7 to 17% in the TiNOX and from 27% to 39% in the control group. Importantly, the difference in MACE between the two groups remained significant (p=0.03; Figure 1) at five year follow-up, mainly driven by the higher need for target lesion revascularisation in the control group (Figure 2).
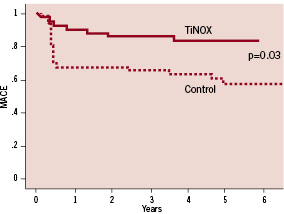
Figure 1. Kaplan-Meier curves for major adverse cardiac events (MACE).
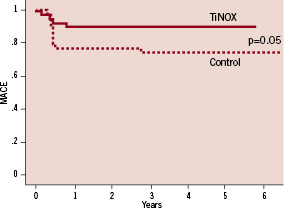
Figure 2. Kaplan-Meier curves for target lesion revascularisation (TLR).
There were no significant differences with respect to myocardial infarction or all cause mortality (Table 3).
Stent thrombosis rate was generally low, and occurred only in one patient of the control group during the first six months. During five years follow-up, no other case of stent thrombosis was observed.
Discussion
In spite of their advantage for prevention of restenosis, the principal short comings of the 1st generation of drug eluting stents (DES) are incomplete vascular healing and late stent thrombosis15-17. The mechanisms are unclear, but due to the lack of re-endothelialisation, stent thrombosis may occur at the site where the stent struts are not covered by a functioning endothelium18,19. Uncovered stent struts bear an inherent risk for stent thrombosis, such as in the case of early after implantation when the stent is not fully expanded or late when there is no vascular healing. This observation has led to the recommendation of the Federal Drug Administration that patients with DES should be treated by dual antiplatelet therapy for at least 12 months, possibly even longer.
Abnormal vasomotion proximal and distal to DES (but not to BMS) has been reported during exercise when coronary flow increases, but when coronary vessels exhibit paradoxical vasoconstriction20-22. This abnormal vasomotion has been attributed to endothelial dysfunction23,24. Another mechanism for late and very late stent thrombosis has been described by Virmani and colleagues25,26 as a hypersensitivity reaction, presumably related to the polymer. A third mechanism has been attributed to the phenomenon of late stent malapposition with negative remodelling of the vessel27.
Titanium-coated stents
Besides the development of 2nd generation DES, another approach to circumvent these problems is the use of passive stent coatings such as titanium-nitride-oxide11,12. This material provides high corrosion resistance and a low tissue reaction associated with enhanced vascular healing11,12,28. Titanium, as a surface coating, has been widely used in medicine, mainly due to its effects on healing and its excellent biocompatibility29,30. We used this material for the coating of the TiNOX stents because initial studies showed excellent results with regard to safety and efficacy. The present study confirms favourable long-term outcome with low MACE rates and no stent thrombosis. The need for revascularisation was 9% in the TiNOX and 25% in the control group with little progression over time.
Clinical implications
Long term follow-up data with the TiNOX stent show a stable clinical course beyond six months after implantation (Figure 1, 2). The initial phase after stent implantation shows low MACE rates, with a few clinical events up to one year mainly driven by the need for revascularisation, but not exceeding 10% of treated patients. All-cause mortality, myocardial infarction and stent thrombosis were rather low, documenting the favourable performance of the TiNOX stent in the long term range. In contrast, the control group with bare metal stents showed a higher need for revascularisation early after implantation (23%) but long term follow-up with regard to all-cause mortality and myocardial infarction were as stable as in the TiNOX group. The major difference between the two stents becomes evident in the early phase (<6 months) after implantation when the bare metal stent elicits a higher rate of MACE, primarily due to the need for target lesion revascularisation (Figure 1).
Medical therapy
Medical therapy is essential for the prevention of late and very late stent thrombosis, but also for the progression of native coronary artery disease. In this regard TiNOX stents have several advantages: First, dual antiplatelet treatment is recommended for 12 months after DES implantation31, whereas TiNOX stents require only one month of thienopyridine therapy. All patients in the present study were treated with aspirin 100 mg and clopidogrel 75 mg daily (after 300 mg loading dose) for one month. Despite the short treatment regimen with dual antiplatelet therapy, only one case of stent thrombosis was encountered, and progression of the coronary artery disease was minimal. This treatment scheme may be particularly advantageous in non-compliant patients, those in need of oral anticoagulation, or in patients at increased risk of bleeding or those requiring extracardiac surgery. Second, the influence of medical therapy (aspirin, statin, ACE inhibitors) on the progression of coronary artery disease may be important, and may explain why a stable long-term outcome was observed in our patients32-37 (Table 2).
Study limitations
This is a small randomised study with 92 patients followed for five years, not powered to address safety endpoints and clinical outcome. Moreover, 5-year follow-up was a secondary endpoint with a 5% loss of follow-up data.
Conclusions
The present long-term follow-up study in patients treated with titanium-nitride-oxide coated stents shows a favourable outcome with an overall MACE rate of 16% and a stable clinical course between one and five years. Despite the short treatment phase with dual antiplatelet therapy of one month, MACE rates were low over the entire observation period, suggesting early vascular healing after stent implantation.

