Titanium-nitride-oxide-coated stents have intriguing biological properties. They are more biocompatible than bare metal stents and reduced neointimal hyperplasia after implantation could be demonstrated in a pig model1. This positive effect could be confirmed in men in a small angiographic follow-up study2. The better tissue acceptance may lead to a faster and more complete endothelialisation after implantation as demonstrated by optical coherence tomography3.
Clinical experience with these devices has shown impressive results in real-life trials (Table 1). In many clinical studies this stent fared better than bare metal stents (BMS) and even paclitaxel-eluting stents in low- and high-risk patient groups4-18. However, in a single centre trial with an angiographic endpoint, late loss after six months was higher in titanium stents than in zotarolimus-eluting stents9.
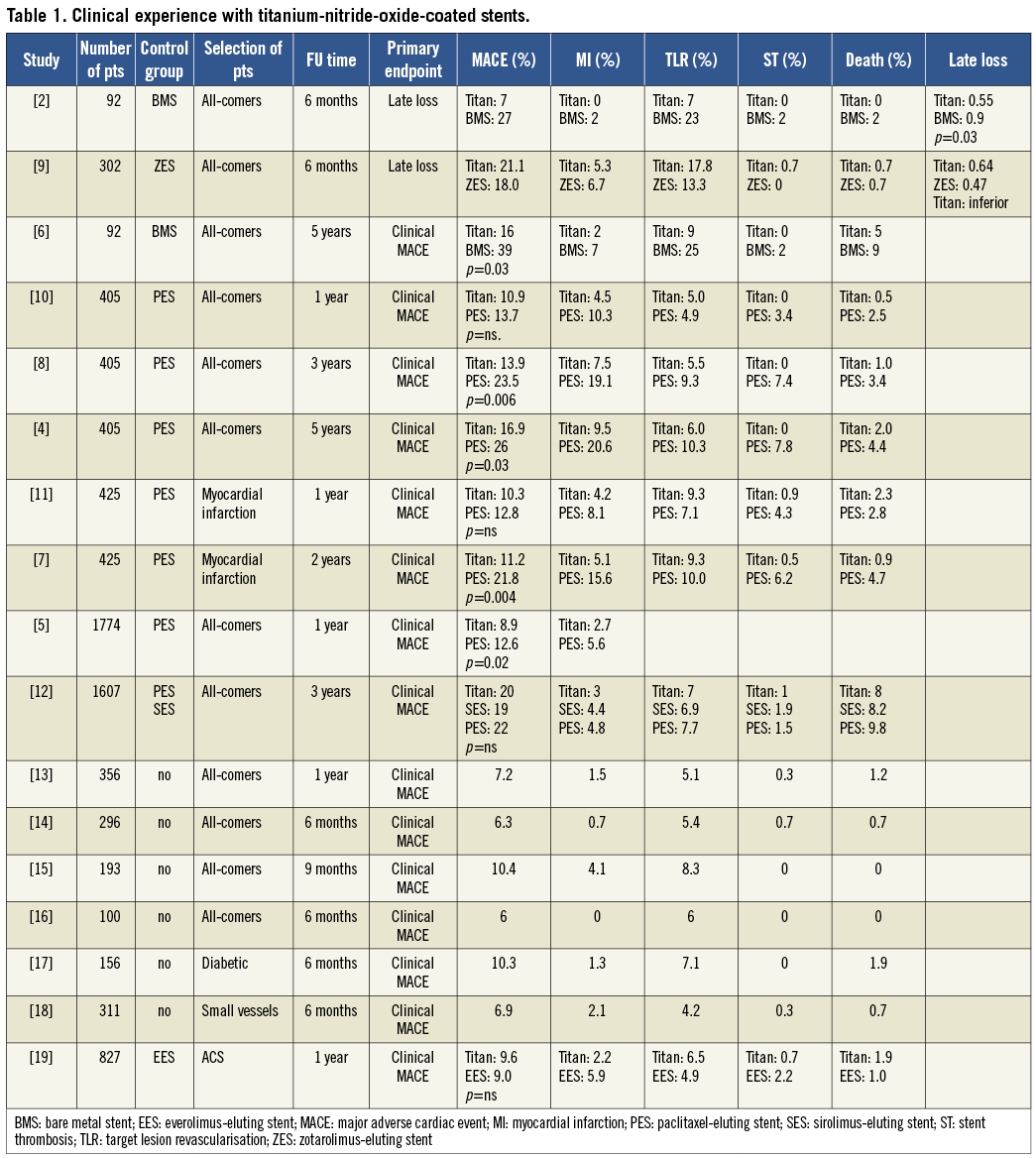
The multicentre trial published in this journal represents an important addition to our knowledge, providing evidence that titanium stents show clinical results comparable to second generation drug-eluting stents (DES) during a follow-up of 12 months, at least in patients with acute coronary syndromes19.
This trial has admittedly been flawed by some methodological inconsistencies including protocol changes, relatively small number of patients, restricted stent length used, non-standardised drug treatment during percutaneous coronary interventions (PCI) and in the follow-up period, especially, the length of double antiplatelet therapy. Nonetheless, it represents an important step in the clinical evaluation of this stent.
The number of trials conducted so far does not suffice to conclude that the Titan stent does have the same safety and efficacy as second generation DES. There are no long-term angiographic results available. Nevertheless, its performance has been encouraging and it might represent a good alternative to DES, because it does not fall into the category that is associated with the need for DAPT.
Further research is needed to define the definite role of this stent in clinical practice and to compare its long-term clinical and angiographic performance with newer generation DES.

