Abstract
Aims: Stents with a passive coating of titanium-nitride-oxide (TiNO) have been compared with Endeavor® zotarolimus-eluting stents (E-ZES) with regard to the primary endpoint of in-stent late lumen loss at six to eight months. The objective of the present analysis was to compare the long-term outcomes of TiNO stents with E-ZES up to five years of clinical follow-up.
Methods and results: A total of 302 patients had been randomly allocated to treatment with TiNO or E-ZES. Up to five years of follow-up, major adverse cardiac events (MACE), the composite of cardiac death, myocardial infarction, or clinically indicated target vessel revascularisation (TLR), were observed in 27.6% of patients treated with TiNO stents and 25.3% of patients treated with E-ZES (RR 1.13, 95% CI: 0.72-1.75, p=0.60), with the majority of events related to clinically indicated TVR (TiNO 21.7% versus E-ZES 20.7%, RR 1.10, 95% CI: 0.67-1.81). There were no differences with respect to individual events including cardiac death, myocardial infarction or stent thrombosis between the two treatment arms up to five years of follow-up. A majority of patients remained free from angina throughout the entire study duration (TiNO 77.3% versus E-ZES 76.1%, p=0.92).
Conclusions: Final five-year outcomes of the TIDE trial comparing TiNO stents with E-ZES revealed increased rates of MACE driven primarily by clinically indicated TVR. The TIDE trial is registered at ClinicalTrials.gov: NCT00492908.
Introduction
Titanium-nitride-oxide-coated (TiNO) stents have been introduced to attenuate the acute inflammatory response to stent-mediated arterial injury and promote arterial healing while maintaining the antirestenotic efficacy. Passive stent coating with TiNO has been shown to decrease platelet adhesion and fibrinogen binding in vitro and to reduce neointimal hyperplasia in a porcine restenosis model1. In a randomised controlled trial, TiNO stents were superior to bare metal stents with regard to late luminal loss at six months and major adverse cardiac events (MACE) at six months and five years2,3. TiNO stents were associated with a lower cumulative rate of MACE at five years of follow-up as compared with paclitaxel-eluting stents (PES) in a randomised controlled trial of patients with acute myocardial infarction4.
In the TIDE trial, powered for a primary angiographic endpoint, TiNO stents were inferior to Endeavor® zotarolimus-eluting stents (E-ZES; Medtronic, Minneapolis, MN, USA) with regard to late loss and binary restenosis5. In the present analysis we present the final five-year clinical outcomes of the TIDE trial.
Methods
PATIENT POPULATION
Inclusion criteria of the TIDE trial have been published previously5. In summary, patients aged ≥18 years presenting with stable angina or unstable angina with at least one lesion with a diameter stenosis of ≥50% were eligible for enrolment. All patients gave written informed consent for participation in the study, which was approved by the local ethics committee. The TIDE trial was an investigator-initiated study supported by the Inselspital Foundation and performed without industry involvement. The study was registered at ClinicalTrials.gov: NCT00492908.
STUDY DESIGN AND PROCEDURES
The design and conduct of the TIDE study, an assessor-blind non-inferiority trial with a primary angiographic endpoint at six to eight months, have been outlined previously5. Patients were randomly assigned to treatment with TiNO stents (Helistent Titan 2; Hexacath, Rueil-Malmaison, France) or E-ZES in a 1:1 fashion in three institutions in Switzerland.
STUDY ENDPOINTS AND DEFINITIONS
The primary endpoint of the study was in-stent late lumen loss at six to eight months after stent implantation as assessed by quantitative coronary angiography and was published previously.
MACE were defined as the composite of cardiac death, myocardial infarction or clinically indicated target vessel revascularisation (TVR). Follow-up was performed by the use of a standardised telephone interview yearly up to five years after intervention.
STATISTICAL ANALYSIS
Baseline clinical characteristics are reported as means and standard deviations and numbers and percentages as appropriate. Cumulative incidences of MACE and clinically indicated target lesion revascularisation (TLR) up to five years are shown by the Kaplan-Meier technique. We used the Mantel-Cox model and the corresponding log-rank test for between-group comparison of clinical outcomes occurring up to five years. All patients who underwent randomisation were included in the analysis in the group to which they were originally allocated (intention-to-treat principle). No adjustments were made for multiple comparisons; all p-values and 95% confidence intervals are two-sided. All analyses were carried out using Stata Version 13 (StataCorp, College Station, TX, USA).
Results
A total of 302 patients had been randomly assigned to treatment with TiNO stents or E-ZES. Baseline clinical characteristics of the two treatment arms were comparable.
Clinical events at five years are summarised in Table 1. There was no difference in the rate of MACE between the two groups at five years (TiNO 27.6% vs. E-ZES 25.3%, RR 1.13, 95% CI: 0.72-1.75, p=0.60). The majority of MACE up to five years occurred within the first year of follow-up and were related to clinically indicated TVR in a majority of cases (Table 1, Figure 1), driven at least in part by protocol-mandated angiographic follow-up. Late events between one and five years were attributable to all-cause mortality, and clinically indicated TVR. TLR accounted for the majority of cases with clinically indicated TVR and is shown up to five years in Figure 2. One case of definite early stent thrombosis occurred in one of the patients treated with TiNO stents. There were no differences with respect to any of the individual outcomes between the two treatment arms up to five years of follow-up (Table 1). More than three quarters of the patients reported being free of angina at five years of follow-up (TiNO 102/152 [77.3%] versus E-ZES 102/150 [76.1%], p=0.89). From 97% at year two, adherence to aspirin decreased to 92% and 95%, respectively, at five years owing to more frequent use of clopidogrel (TiNO 11.9% vs. E-ZES 7.3%, p=0.22) (Table 2).
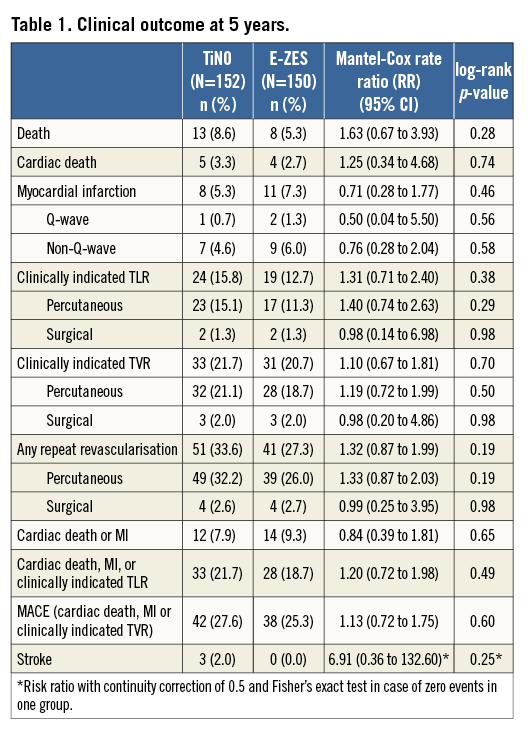
Figure 1. Major adverse cardiac events (composite of cardiac death, myocardial infarction or clinically indicated target vessel revascularisation). Cumulative event curves show the composite endpoint of cardiac death, myocardial infarction, and clinically indicated target vessel revascularisation up to five years of follow-up for TiNO stents and E-ZES.
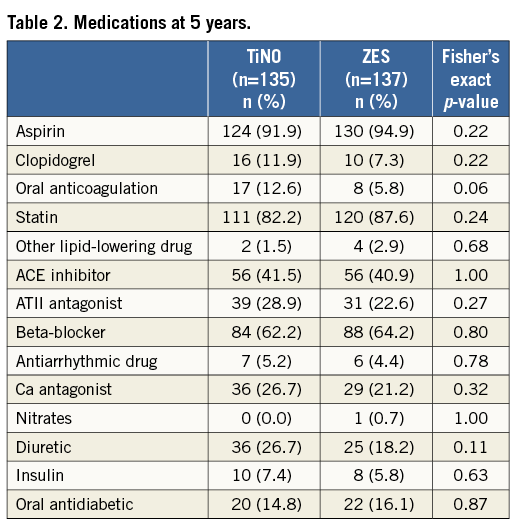
Figure 2. Clinically indicated target lesion revascularisation. Cumulative event curves show the clinically indicated target lesion revascularisations up to five years of follow-up for TiNO stents and E-ZES.
Discussion
The key messages of the final five-year clinical outcome of the randomised comparison between the TiNO stent and the E-ZES are the following. 1) There was no significant difference with regard to MACE between patients treated with the TiNO stent and the E-ZES up to five years of follow-up. 2) The majority of MACE in both treatment arms occurred within the first year after intervention and were frequently associated with clinically indicated TVR.
The present study has several limitations. First, the trial was powered for a primary angiographic endpoint and is hence underpowered to evaluate differences between TiNO and E-ZES with regard to clinical outcome. Second, protocol-mandated angiographic follow-up at six to eight months increased the rates of revascularisation procedures disproportionately. Third, both stent types have been superseded by novel stent technologies with improved safety and efficacy profiles. E-ZES has largely been replaced by the Endeavor Resolute DES (Medtronic), characterised by delayed and more sustained drug elution related to a novel polymer. However, the recent publication of the final results of the SORT OUT III trial emphasise the importance of long-term clinical outcomes in coronary stent trials to elucidate fully their safety and efficacy profiles6.
The rate of MACE up to five years was considerably higher in the TIDE trial (27.6%) as compared to the event rates reported in the PORI registry (16.9%)7 and even the TITAX AMI trial4. An “oculo-stenotic reflex” during the protocol-mandated repeat angiography may account for a difference in TLR, along with a high proportion of complex patients and longer lesion length compared to previous trials. The difference in clinical event rates between the TiNO stent and the E-ZES appeared to diminish during the course of the long-term follow-up.
The present trial was designed in the era of early-generation DES when long-term safety of DES had just been raised as a concern. With the introduction of newer-generation DES with high antirestenotic efficacy, improved strut endothelialisation, shortened minimal duration of dual antiplatelet therapy, and low rates of stent thrombosis, the role of passive stent coatings remains to be determined. Thin-strut DES releasing antiproliferative agents from biocompatible or biodegradable polymers demonstrated a rate of TLR ranging from 2% to 4% in recent trials8,9 and had negligible rates of very late ST.
Conclusion
The final five-year outcomes of the TIDE trial comparing TiNO stents with E-ZES revealed increased rates of MACE driven primarily by clinically indicated TVR.
| Impact on daily practice Percutaneous coronary intervention with TiNO and E-ZES is associated with increased rates of MACE driven primarily by clinically indicated TVR. Both devices have been overtaken by newer iterations of drug-eluting stents. |
Conflict of interest statement
T. Pilgrim has received lecture fees from Biotronik and Medtronic. P. Wenaweser has received a research grant to the institution from Medtronic, lecture and proctoring fees from Medtronic, Edwards, and Boston Scientific. P. Jüni is an unpaid steering committee or statistical executive committee member of trials funded by Abbott Vascular, Biosensors, Medtronic, and Johnson & Johnson. CTU Bern, which is part of the University of Bern, has a staff policy of not accepting honoraria or consultancy fees. However, CTU Bern is involved in the design, conduct, or analysis of clinical studies funded by Abbott Vascular, Ablynx, Amgen, AstraZeneca, Biosensors, Biotronik, Boehringer Ingelheim, Eisai, Eli Lilly, Exelixis, Geron, Gilead Sciences, Nestle, Novartis, Novo Nordisc, Padma, Roche, Schering-Plough, St. Jude Medical, and Swiss Cardio Technologies. S. Windecker has received research contracts to the institution from Biotronik and St. Jude.

