Abstract
Aims: To evaluate whether the improved outcomes with newer generation drug-eluting stents (DES) utilising thin-strut stents are consistent among different patient and angiographic subgroups.
Methods and results: Clinical outcomes over three years were collected in the SPIRIT III trial comparing the XIENCE V® everolimus-eluting stents (EES) (Abbott Vascular, Santa Clara, CA, USA) to the TAXUS® paclitaxel-eluting stents (PES) (Boston Scientific, Natick, MA, USA). Potential predictors of adverse clinical outcomes were assessed using demographic, clinical, and procedural variables by logistic and Cox regression analyses. For three-year target vessel failure, the independent predictors identified by Cox regression were number of vessels treated (HR=2.19 [1.50, 3.19], p<0.0001), HbA1c (%) (HR=1.17 [1.05, 1.29], p=0.004), total cholesterol (>200 mg/dl) (HR=1.63 [1.13, 2.36], p=0.009), and female gender (HR=1.42 [1.01, 2.01], p=0.05). Logistic regression analysis identified the same predictors except for the female gender. The clinical results with EES compared to PES were consistent among the multiple subgroups examined with the possible exception of patients with diabetes.
Conclusions: Clinical factors and stent type were the most important multivariable predictors of adverse clinical outcomes in this contemporary trial of first versus second generation DES. The benefit of the EES compared to the PES was consistent across a wide range of patient and angiographic subgroups with the possible exception of patients with diabetes.
Abbreviations
EES: everolimus-eluting stent
MACE: major adverse cardiac event
MI: myocardial infarction
PCI: percutaneous coronary intervention
PES: paclitaxel-eluting stent
TLR: target lesion revascularisation
TVF: target vessel failure
TVR: target vessel revascularisation
ZES: zotarolimus-eluting stent
Introduction
Drug-eluting stents (DES) have dramatically reduced the rates of restenosis and the need for repeat revascularisation in patients undergoing percutaneous coronary intervention (PCI) for symptomatic coronary artery disease1,2. However, the rates of event-free survival after DES implantation vary among patient and lesion subgroups. Patient risk factors including older age, diabetes, hypertension and chronic kidney disease are associated with mortality and adverse cardiovascular events, and procedural factors such as small vessel size, longer lesions, and multiple and overlapping stents have been associated with higher rates of clinical restenosis3,4. Whether differences in event-free survival exist between first generation sirolimus-eluting and paclitaxel-eluting stents has been the subject of debate and numerous studies.5
Current coronary DES systems consist of a metallic stent backbone, durable polymer and antiproliferative drug. Each of these components may affect device safety and efficacy, although isolating the effect of each component to outcomes is problematic. Newer generations of DES have incorporated stents with thinner struts, more biocompatible and less potentially inflammatory polymers, and lower drug dosages in an effort to minimise stent-associated late clinical events such as stent thrombosis, which may arise from delayed or poor vessel healing. The large-scale SPIRIT III randomised controlled clinical trial compared clinical and angiographic outcomes from use of a TAXUS® EXPRESS2® paclitaxel-eluting stent (PES) (Boston Scientific, Natick, MA, USA) to that of a thinner strut XIENCE V® everolimus-eluting stent (EES) (Abbott Vascular, Santa Clara, CA, USA).6 At nine months, and at two and three years, the EES resulted in lower major adverse event rates than the PES6-8. Whether this benefit extends to all subgroups of patients enrolled in the study, however, remains uncertain. Therefore, the current analysis was performed to identify the independent predictors of outcomes in the SPIRIT III trial.
Methods
The design of the SPIRIT III trial has been previously described6. In brief, SPIRIT III was a prospective, multicentre, randomised, single-blind, controlled clinical study in which 1,002 patients with up to two de novo lesions in native coronary arteries with a reference vessel diameter (RVD) of 2.5-3.75 mm and lesion length ≤28 mm were randomised in a 2:1 ratio to receive either the EES (XIENCE V®) or the PES (TAXUS® EXPRESS2®)
Inclusion criteria
Enrolment was restricted to patients ≥18 years of age with stable or unstable angina or inducible ischaemia. Patients who met the clinical eligibility criteria provided signed informed consent prior to enrolment. Eligibility of the patient to be enrolled in the study was confirmed based on the angiogram just before the intended procedure (the pre-procedure angiography). Key angiographic inclusion criteria included a maximum of two de novo native coronary artery lesions, each in a different epicardial vessel, RVD of ≥2.5 mm and ≤3.75 mm, lesion length ≤28 mm by visual estimation, % diameter stenosis (%DS) of ≥50% and <100%, TIMI flow of ≥1, non-target vessel percutaneous intervention in non-target vessel planned ≥90days prior to or >9months after the index procedure.
Exclusion criteria
Major clinical exclusion criteria included PCI in the target vessel prior to or planned within nine months of the index procedure, or in a non-target vessel within nine months of index procedure; acute or recent myocardial infarction (MI); left ventricular ejection fraction <30%; use of chronic anticoagulation or immunosuppressive therapy; known autoimmune disease, renal insufficiency, recent major bleed, haemorrhagic diathesis or objection to blood transfusions; contraindications or allergy to any of the study medications, components of the study stents, or iodinated contrast that could not be pre-medicated; elective surgery planned within nine months after the procedure necessitating antiplatelet agent discontinuation; platelet count <100,000cells/mm3 or >700,000 cells/mm3, white blood cell count <3,000 cells/mm3, serum creatinine >2.5mg/dL or dialysis, or liver disease; stroke or transient ischaemic attack within six months; comorbid conditions limiting life expectancy to less than one year or that could affect protocol compliance; and participation in another investigational study that had not yet reached its primary endpoint.
Key angiographic exclusion criteria included aorto-ostial location, left main location, bifurcation involvement, excessive tortuosity, extreme angulation (≥90°), heavy calcification, target vessel containing thrombus, other significant lesions (>40% DS) in the target vessel or side branch for which intervention was required within nine months. If two target lesions were treated, each of these lesions had to meet all angiographic inclusion/exclusion criteria.
Randomisation and blinding
Randomisation could only take place after verification of the inclusion/exclusion criteria and successful predilatation of the first target lesion. Following confirmation of all general and angiographic eligibility, telephone randomisation to EES vs. PES was performed as previously described6. Although the operators were by necessity unblinded during the stent implant procedure, the patient and staff involved in follow-up assessments remained blinded until all patients completed the primary endpoint follow-up, with a standardised follow-up interview script used to reduce bias. A patient was considered enrolled in the study from the moment he or she had been randomised.
Interventional procedure
EES were available in 2.5, 3.0, and 3.5 mm diameters, and in 8, 18, and 28 mm lengths. The full range of US-manufactured PES was available, ranging from 2.5 to 3.5mm in diameter and from 8 to 32mm in length. The stent selected was of sufficient length to cover approximately 3mm of non-diseased tissue on either side of the lesion. In patients receiving multiple stents for a single lesion, 1mm to 4mm of stent overlap was recommended. Post-dilatation was left to the discretion of the investigator; and if performed, it was only done with balloons sized to fit within the boundaries of the stent. If an additional stent was needed for bailout purposes, it was required to be from the same treatment arm as the first implanted stent.
Patients who were not on chronic antiplatelet or aspirin therapy were required to receive aspirin ≥300mg pre-procedure and a loading dose of clopidogrel bisulfate ≥300mg no later than one hour after the procedure. All patients were to be maintained on 75mg clopidogrel bisulfate daily for a minimum of six months and ≥80mg of aspirin daily throughout the length of the trial (five years) following the index procedure. Subjects who developed hypersensitivity to clopidogrel bisulfate were switched to ticlopidine hydrochloride at a dose in accordance with standard hospital practice.
Stent delivery system
The XIENCE V EES, evaluated in the SPIRIT trials, consisted of either the L-605 cobalt chromium (CoCr) alloy-coated MULTI-LINK VISION or MULTI-LINK MINI VISION stent mounted on a MULTI-LINK RX VISION or MULTI-LINK MINI VISION RX delivery system, respectively. All stent diameters were available in 8-28mm lengths. The coating consisted of two layers: a primer layer and a drug matrix layer. The EES contains 100µg/cm2 of everolimus, which is released from a thin (7.8μm), non-adhesive, durable, biocompatible fluoropolymer coated onto a low profile (0.0032” [0.0813mm] strut thickness), flexible cobalt-chromium stent6.
Statistical methods for clinical results
For the intent-to-treat population, univariable and multivariable logistic and Cox regression analyses were used to identify significant predictors of nine-month and three-year target vessel failure (TVF: cardiac death, myocardial infarction [MI], or ischaemia-driven target vessel revascularisation [TVR]) and major adverse cardiac events (MACE: cardiac death, MI, or ischaemia-driven target lesion revascularisation [TLR]). These two pre-specified composite endpoints were used as the measures of interest for the present post hoc analysis as they occurred with the highest frequency of all clinically-assessed outcome measures, providing more power for multivariable and subgroup analyses. Univariable and multivariable logistic and Cox regression analyses were also used to identify predictors of nine-month and three-year cardiac death or MI and TLR, which were used as separate measures of safety and clinical efficacy. Demographic, clinical and procedural variables were included in these analyses. Cox regression analyses were performed to identify significant predictors of nine-month and three-year outcomes (TVF, MACE, cardiac death or MI, and TLR) to adjust the follow-up duration through the time-to-event nature of the model.
For the univariable analyses, coefficients, Wald chi-square p-values, odds ratios (or hazard ratio from the Cox model), and 95% confidence intervals for the odds ratios (or hazard ratio) were displayed. For the multivariable analyses, models were built using a stepwise (forward/backward) elimination procedure, with independent variables entered into the model at the 0.20 significance level and removed at the 0.05 level. A final model was selected and presented with selection criteria based on both statistical significance and clinical consideration, with the exception of stent treatment type, which was forced into the final model of the multivariable regression analysis. Displayed for each variable in the final multivariable model were the final coefficient, the standard error for the coefficient, and the p-value from the Wald chi-square, the odds ratios (or hazard ratio from the Cox model), and 95% confidence intervals for the odds ratios (or hazard ratio). In addition, the adequacy of the fitted model was displayed in the multivariable logistic regression analysis and was evaluated by the Hosmer and Lemeshow goodness-of-fit test and c-statistic, which is the area under the receiver operating characteristic (ROC) curve. A score test for proportionality based on scaled Schoenfeld residuals was also performed to assess the proportional hazards assumption for the Cox proportional hazards model. All statistical analyses were performed in the intention to treat population by using SAS version 9.1.3 (SAS Institute Inc, Cary, NC, USA).
Results
The rates of TVF and MACE at nine months and three years are shown in Figure1. At nine months, clinical follow-up was available in 980 patients (97.8%), including 658 EES patients and 322 PES patients. There was no significant difference in the rates of TVF between EES and PES (7.6% vs. 9.7%, respectively; p=0.27). MACE was significantly reduced by 43% in the EES cohort compared to the PES cohort (5.0% vs. 8.8%, respectively: p=0.036). Clinical follow-up at three years was available in 948 patients (94.6%), including 636 EES patients and 312 PES patients. At three years, TVF occurred significantly less frequently with EES as compared with the PES (14.3% vs. 20.0%, respectively; p=0.03), as did MACE (9.7% vs. 16.4%, respectively; p=0.004).
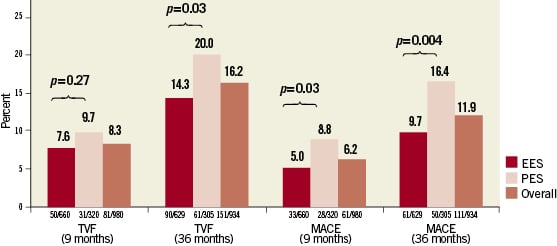
Figure 1. Bar graph of percent incidence of target vessel failure (TVF) and major adverse cardiac events (MACE) at nine months and three years for everolimus-eluting stents (EES) and paclitaxel-eluting stents (PES).
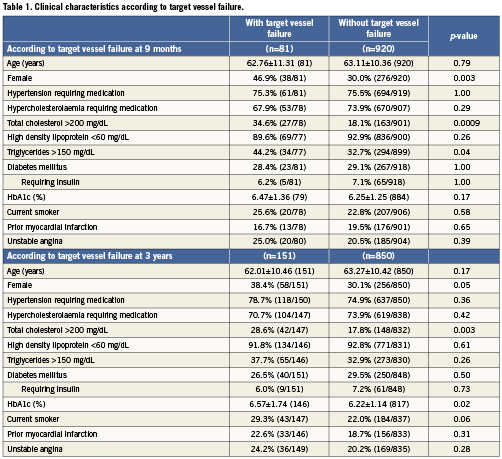
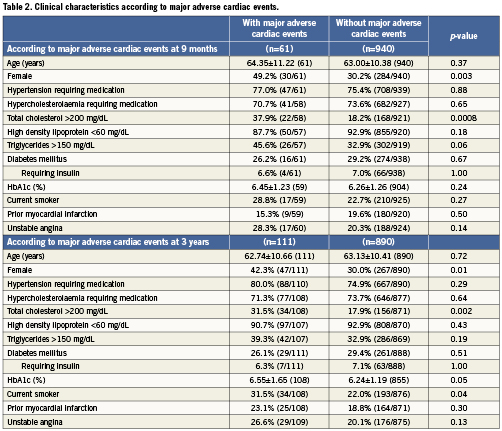
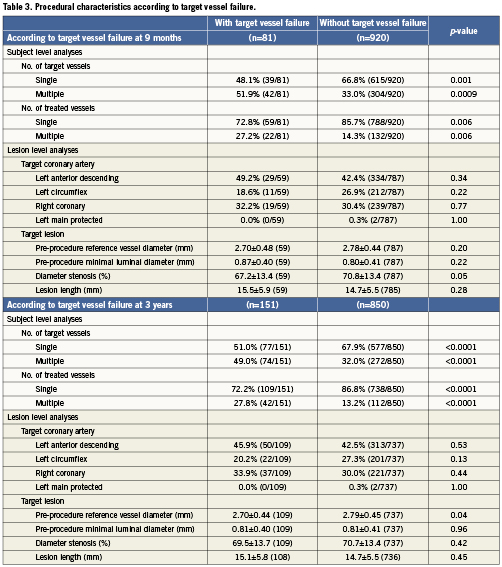
Baseline clinical characteristics associated with TVF are shown in Table1. At nine-month follow-up, female gender, total cholesterol >200mg/dL, and triglycerides >150mg/dL were associated with TVF. At three-year follow-up, haemoglobin A1c (HbA1c) (%) was associated with TVF in addition to female gender and total cholesterol >200mg/dL. As shown in Table2, female gender and total cholesterol >200 mg/dL were also associated with an increased risk of MACE at both nine months and three years. HbA1c (%) and current smoking were associated with MACE at three-year follow-up. Procedural characteristics associated with TVF (Table 3) and MACE (Table 4) included the presence of multivessel disease at both nine months and three years and smaller pre-procedure RVD at three years.
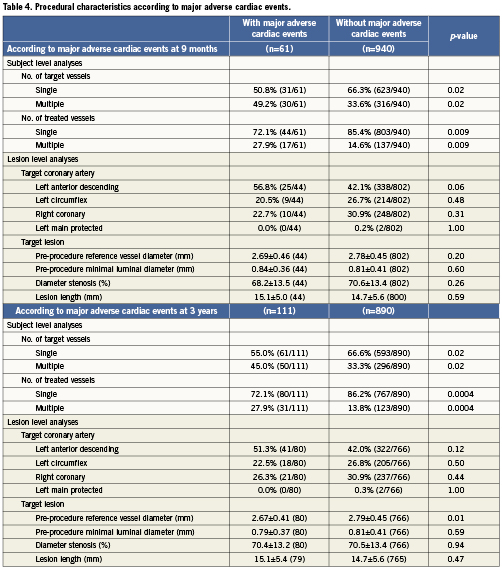
Multivariable analysis
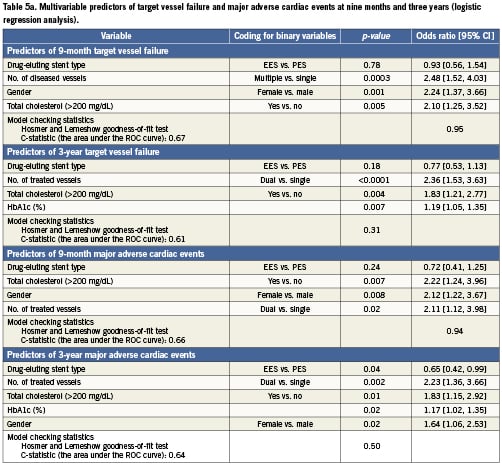
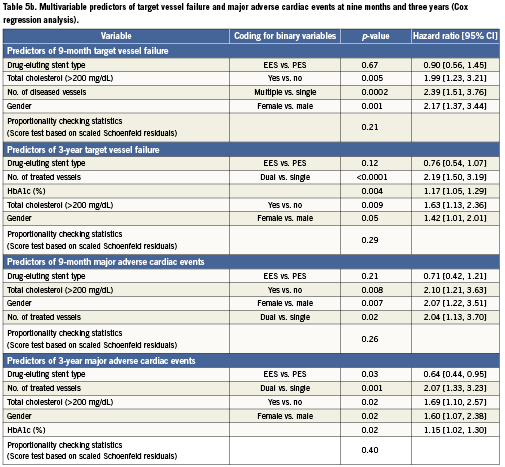
The predictors of composite endpoints of TVF and MACE at nine months and three years are shown in Table5 from both logistic (Table 5a) and Cox (Table 5b) regression analyses. These two statistical methods produced very similar results. At nine months (logistic regression analysis), the number of diseased vessels, female gender, and total cholesterol >200mg/dL were identified as predictors of TVF. At three years (Cox regression analysis), the predictors of TVF included the number of vessels treated, HbA1c (%), total cholesterol >200mg/dL, and female gender. At nine months (logistic regression analysis), the predictors of MACE were total cholesterol >200mg/dL, female gender, and number of vessels treated. At three years (Cox regression analysis), the predictors of MACE were the number of vessels treated, total cholesterol >200mg/dL, female gender, and HbA1c (%). Stent type (use of EES rather than PES) was a significant predictor of lower MACE at three years (p=0.03; HR=0.64 [95% CI, 0.44-0.95]).
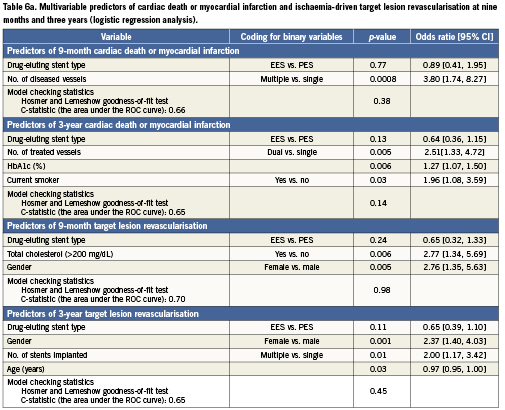
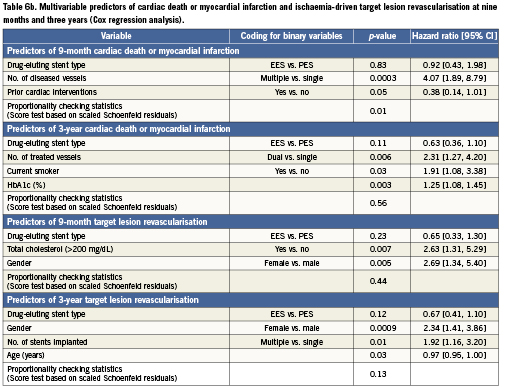
The predictors of cardiac death or MI (safety) and ischaemia-driven TLR (clinical efficacy) at nine months and three years are shown in Table6 from both logistic (Table 6a) and Cox (Table 6b) regression analyses. The predictors of cardiac death or MI at nine months (logistic regression analysis) included the number of diseased vessels and prior cardiac intervention, and at three years (Cox regression analysis) were the number of vessels treated, current tobacco use, and HbA1c (%). The predictors of TLR were female gender and total cholesterol >200mg/dL at nine months (logistic regression analysis), and female gender, number of stents implanted and age at three years (Cox regression analysis).
Subgroup interaction analysis
Implantation of EES as compared with PES reduced the rate of TVF at three years, and MACE at both nine months and three years. To determine if there was an interaction between DES type and clinical and procedural factors, we evaluated the relative risk of selected clinical and procedural characteristics, as well as angiographic results stratified by EES vs. PES, for TVF and for MACE at three years. There were no significant interactions between the subgroups and stent randomisation for the occurrence of TVF or MACE at three years.
Discussion
The present study evaluated the independent predictors of TVF and MACE at nine months and three years after DES placement in a contemporary group of patients. Results from logistic and Cox regression analyses were similar due to the relatively high three-year follow-up rates in the SPIRIT III (94.6%) trial. Clinical variables including female gender, total cholesterol >200mg/dL, and HbA1c were independent predictors of both TVF and MACE. Procedural factor treatment of dual vessels were predictors of clinical outcomes at two years9 and remained an independent predictor of TVF and MACE. Finally, stent type (use of EES rather than PES) was also a multivariable predictor of lower MACE at three years. These observations provide evidence that demographic, clinical, and procedural characteristics remain important factors in determining clinical outcomes in the contemporary DES era. The benefits of EES compared to PES were present across a broad range of clinical and angiographic subgroups including those identified as predictors of higher adverse outcomes at three years in multivariable regression analysis, with the possible exception of patients with diabetes mellitus in whom the outcomes of EES and PES were not significantly different. These data should provide reassurance that EES, one of the newest generations of DES, provides consistent and durable benefits for up to three years across a broad range of clinical and procedural subgroups.
A baseline total cholesterol of >200mg/dL emerged as a multivariable clinical predictor of TVF and MACE at both nine months and three years, despite the observation that almost three-fourths of all patients were receiving therapy for hypercholesterolaemia. Given the contemporary clinical emphasis on the aggressive management of hypercholesterolaemia, the observation that total cholesterol >200mg/dL was associated with adverse events may identify patients who are relatively resistant to statins, or have more severe forms of hyperlipidaemia. Also, female gender emerged as another multivariable predictor of TVF and MACE at both nine months and three years. Female gender has been variably identified as an adverse clinical characteristic following percutaneous coronary revascularisation procedures10-14. More recent studies evaluating outcomes after DES use have not identified female gender as an adverse factor affecting clinical or angiographic restenosis13. Our observation that EES use vs. PES use was associated with a substantially lower risk of TVF or MACE at three years in women (with no gender-specific interactions present) is supported by observations from the SPIRIT II and III trials15,16, as well as the one year outcomes of the larger SPIRIT IV trial, which demonstrated a 47% reduction in target lesion failure in women with EES compared to PES17. These observations together suggest that the type of stent used may be an important determinant of clinical outcomes in women.
Considerable debate has arisen over the relative benefit between different DES in patients with diabetes18-20. In the SPIRIT III randomised trial, no difference was observed in the overall rates of TVF or MACE between EES or PES in the subgroup of patients with diabetes6-8, consistent with the results of the recent meta-analyses19,20. Despite the similarity of clinical outcomes in this trial between EES and PES in the diabetic subgroup, HbA1c emerged as an important multivariable predictor of both TVF and MACE at three years in the overall study population. This observation is at odds with recent evaluations that did not find that diabetes was a multivariable predictor of clinical restenosis in all patients4. Several factors may be important in explaining potential reasons for this apparent dichotomy. First, we examined HbA1c as a continuous variable, and in all study patients, not just diabetics, which had not been previously done. It is possible that HbA1c is a more sensitive measure of the effect of diabetes on clinical outcomes than is obtained by simple assessment of the presence or absence of the disease itself. Second, previous studies used restenosis as an endpoint, whereas we evaluated broader clinical outcomes that included MI and death. These two different endpoints represent the consequences of different pathophysiologic processes, restenosis vs. the clinical expression of atherosclerosis, which are likely affected differently by diabetes. In support of this, HbA1c was a multivariable predictor of cardiac death or MI (safety) but not a multivariable predictor of TLR (clinical efficacy) at three years in the SPIRIT III trial. Our observations support the inclusion of HbA1c as a clinical endpoint in future trials of stent therapy.
The observation that EES use was a significant predictor of lower event rates is consistent with prior animal and clinical studies that have evaluated rates of restenosis following the use of thinner strut BMS compared to thicker strut BMS21-23. However, no difference in rates of clinical restenosis or clinical outcomes between a thin-strut zotarolimus-eluting stent (ZES) and PES was noted in a large randomised trial24,25. Both the EES and the ZES system are based on thin-strut cobalt chromium stent platforms, however the drug delivery kinetics of the two stent systems are substantially different, with the majority of zotarolimus released within 14 days from the ZES compared to 30 days observed with the release of everolimus from the EES platform26-28. Differences in vascular responses to the varying polymers may also explain the disparate outcomes between stent types.
Finally, these comparisons are based on the use of the older generation TAXUS EXPRESS platform, which has been replaced by the thin-strut TAXUS® Liberté® (Boston Scientific). It could be argued, therefore, that the comparisons to TAXUS EXPRESS are of historical interest. However, the clinical outcomes of the XIENCE and TAXUS Liberté DES have been compared in the single centre randomised COMPARE trial29. In this “all comers” trial, EES use was associated with a relative risk of the primary endpoint MACE of 0.69 (95% CI, 0.50-0.95; p=0.02) compared to PES. This magnitude of benefit of EES compared to PES is comparable to that observed in the SPIRIT IV trial at one year17. Thus use of EES appears to be superior to that of PES independent of the stent platform. Ultimately, further studies will be needed to confirm the results of the COMPARE trial.
There are limitations of this study that merit discussion. This is a post hoc evaluation of the clinical outcomes from the SPIRIT III study, and thus may be subject to bias. However, there were no significant differences in the clinical and angiographic variables of either stent group so it is unlikely that selection bias significantly affected the study results. Likewise, rates of angiographic and clinical follow-up were excellent and similar in both groups so it is unlikely that ascertainment bias affected the study outcomes. Additionally, while it was interesting to look at the multivariable predictors of cardiac death or MI and TLR, SPIRIT III was not powered for these relatively low frequency events when considered separately. Similar analyses of the larger DES trails such as SPIRIT IV might be able to provide more insights. Finally, patients in this randomised clinical trial were carefully selected with explicit criteria that excluded several high-risk groups of patients and lesions. Thus, these observations may not be generalised beyond the current study population.
Conflict of interest statement
R. Applegate reports having received research support from Cordis and Abbott Vascular and serving on the scientific advisory board for Abbott Vascular; J. Hermiller reports serving as a consultant for Abbott Vascular and Boston Scientific Corp.; P. Gordon reports having received research support from Abbott Vascular; G. Stone reports serving on the scientific advisory board for Abbott Vascular and Boston Scientific Corp.; K. Sudhir, M. Yaqub, P. Sood, X. Su and S. Cao are full-time employees of Abbott Vascular and own stock and/or options of Abbott Vascular.

