Abstract
Aims: The purpose of this study was to investigate the vascular response of the everolimus-eluting stent (EES) compared with the paclitaxel-eluting stent (PES) using serial intravascular ultrasound (IVUS).
Methods and results: Data were obtained from the SPIRIT III trial, a multicentre, 2:1 randomised, controlled study comparing EES and PES in de novo native coronary artery lesions. IVUS images were eligible for volumetric analysis at eight-month follow-up in 158 lesions (EES: 113, PES: 45). At eight months, EES had a smaller neointimal volume index (VI: mm3/mm) (EES: 0.4±0.4 vs. PES: 0.8±0.8 mm3/mm, p=0.002) and also a smaller % neointimal obstruction (EES: 7.1±6.7% vs. PES: 11.1±10.5%, p=0.005) compared with PES. While there was no significant change in vessel VI with EES, there was a significant increase in vessel VI in PES during eight-month follow-up (EES: 0.1±1.2 vs. PES: 1.2±0.8 mm3/mm, p=0.001). There were no statistical differences in the frequency of edge dissection or incomplete stent apposition between the two groups.
Conclusions: Detailed IVUS analysis confirmed significantly less neointimal hyperplasia with EES compared with PES. While there was no increase in vessel volume with EES during the eight-month follow-up period, vessel enlargement was seen at the stented segment in PES.
Introduction
Recently, the everolimus-eluting fluoropolymer-coated XIENCE V® stent (EES) (Abbott Vascular, Santa Clara, CA, USA) demonstrated significant reductions in angiographic late loss with non-inferior rates of target vessel failure and fewer major adverse cardiac events (MACE) compared with the paclitaxel-eluting TAXUS® Express2™ stent (PES) (Boston Scientific, Natick, MA, USA) in the SPIRIT III randomised controlled trial.1 The aim of this intravascular ultrasound (IVUS) substudy was to examine detailed vascular responses to EES compared with PES using serial IVUS analysis.
Methods
STUDY DESIGN AND PATIENT POPULATION
The SPIRIT III global trial consisted of three IVUS cohorts: US registry for randomised controlled trials (RCT), US 4.0 mm EES registry and Japan EES registry. In this study, data were derived from the SPIRIT III RCT trial whose methods have been previously described.1 In brief, this was a prospective, multicentre, single-blind, 2:1 randomised controlled trial to compare the efficacy and safety of EES as compared to PES in de novo native coronary artery lesions. The study protocol was approved by the institutional review board at each investigational site, and consecutive, eligible patients signed the written informed consent.
IVUS PROCEDURE AND ANALYSIS
IVUS interrogation was planned for all patients at pre-specified enrolment sites at post-procedure and at eight months after stent implantation. The IVUS procedure was performed in a standard fashion using automated motorised pullback (0.5 mm/sec) with commercially available systems (40-MHz IVUS catheter; Boston Scientific Corp, Natick, MA, USA; or 20-MHz IVUS catheter; Volcano Corp, Rancho Cordova, CA,USA) at each site. IVUS analyses were done in an independent core laboratory at Stanford University Medical Center (Cardiovascular Core Analysis Laboratory, Stanford, CA, USA).
Volumetric measurements were performed using PC-based software (echoPlaque; Indec Systems Inc., Santa Clara, CA, USA) as previously described.2 Peri-stent plaque volume was calculated as vessel minus stent volume. Neointimal volume was calculated as stent minus lumen volume, and % neointimal obstruction (the overall degree of neointimal proliferation throughout the stented segment) was defined as neointimal volume divided by stent volume. Each volume was divided by measurement stent length to adjust for different stent length (volume index: VI, mm3/mm). Cross-sectional narrowing (CSN, %; the most severe impact of neointima on luminal encroachment) was defined as neointima area divided by stent area. Neointima-free frame ratio (%) was calculated as the number of frames without IVUS-detectable neointima divided by the total number of frames within the stent.3
Stent edge dissection, tissue prolapse, and incomplete stent apposition (ISA) were assessed as qualitative IVUS parameters. ISA was identified as one or more stent strut clearly separated from the vessel wall with evidence of blood speckle behind the strut. ISA was classified as persistent, resolved, or late-acquired as previously described.4,5 All images were reviewed by two independent observers, and adjudication of opinion was based on the consensus of these observers.
Statistical analysis
Statistical analysis was performed using Statview 5.0 (SAS Institute Inc., Cary, NC, USA) software. Continuous variables are expressed as mean±standard deviation. For continuous variables, comparisons between EES and PES were performed with two-tailed, unpaired t-tests; and comparisons between baseline and follow-up were done by paired t-tests. Categorical variables were compared using the chi-square test. A p-value <0.05 was considered statistically significant.
Results
STUDY POPULATION AND BASELINE CHARACTERISTICS
Of the 240 lesions in 210 patients who performed for the eight-month IVUS, 161 lesions (EES: 115, PES: 46) were available for serial qualitative analyses (baseline and follow-up). Volumetric analysis was performed only on IVUS images with a consistent pullback and adequate image quality for every millimetre throughout the stent. After excluding lesions for inadequate pullback or poor image quality, volumetric analysis was possible in 158 lesions (EES: 113, PES: 45) at follow-up and 95 lesions (EES: 69, PES: 26) for serial analyses (Figure 1).
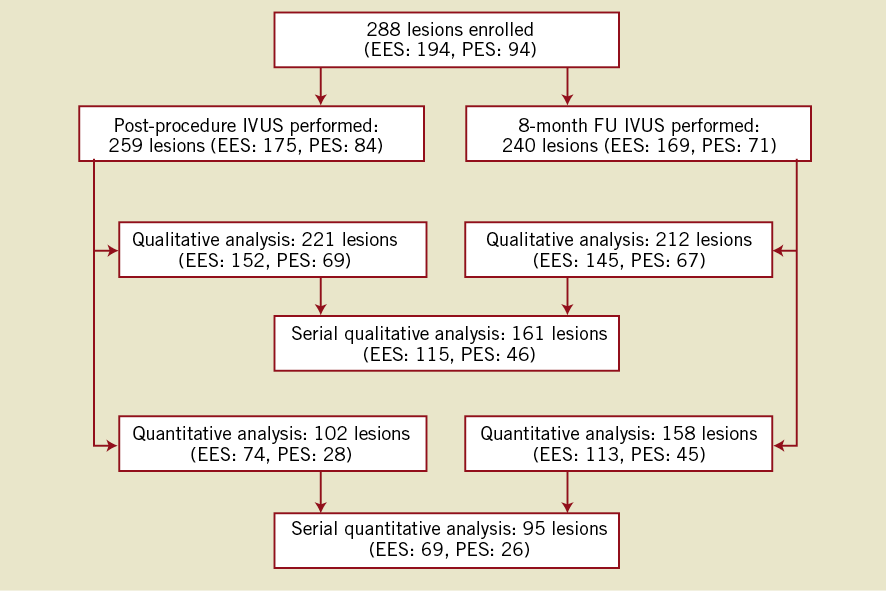
Figure 1. Patient flow-chart. A total of 288 lesions were enrolled in this IVUS substudy, and the core lab received 259 lesions for post-procedure and 240 lesions for follow-up. After excluding cases with poor image quality (post-procedure: 38 lesions; follow-up: 28 lesions), qualitative analyses were available for 221 lesions in post-procedure and 145 lesions in follow-up. For the quantitative (volumetric) analyses, more patients were excluded due to inconsistent pullback and/or missing calibration information. Finally 161 lesions were available for serial qualitative analyses and 95 lesions were available for quantitative analyses.
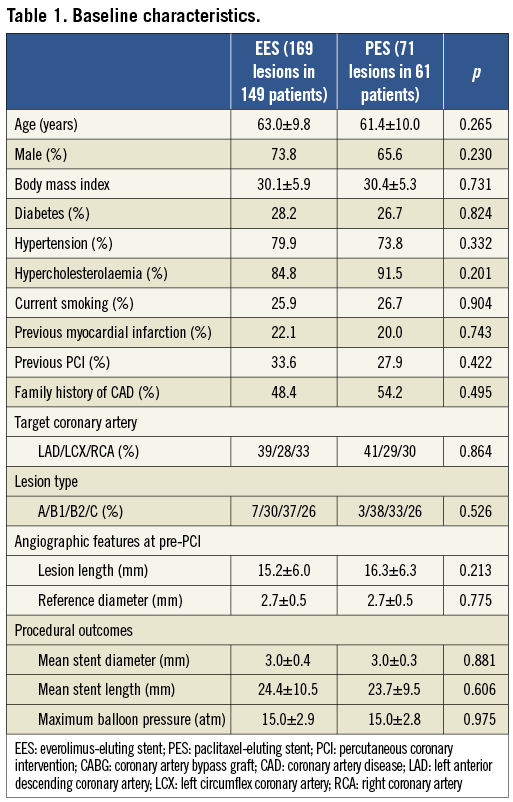
There was no significant difference in baseline characteristics including lesion and procedural characteristics between EES and PES (Table 1).
QUANTITATIVE IVUS ANALYSIS
Quantitative IVUS results of neointimal characteristics are summarised in Table 2, showing significantly smaller neointimal VI, % neointimal obstruction, and maximum CSN in EES compared to PES. The range of % neointimal obstruction was relatively narrower and the distribution was shifted to the left in EES compared to PES (Figure 2).
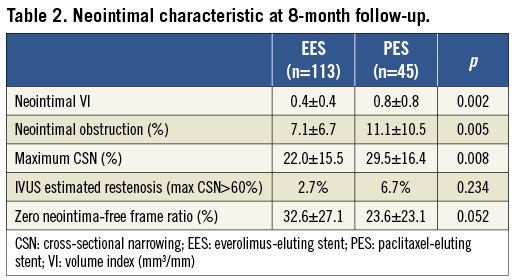
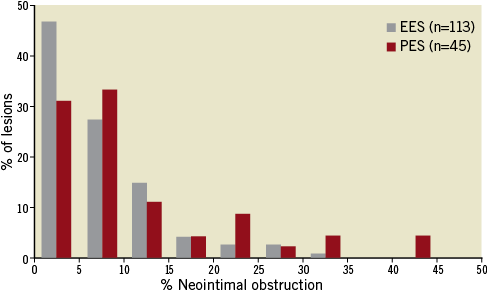
Figure 2. Statistical distribution of neointimal obstruction at 8-month follow-up. The range of % neointimal obstruction was relatively narrower and the distribution was shifted to the left in EES compared to PES.
Serial quantitative IVUS results at the stented segment and adjacent reference segments are listed in Table 3. At the stented segment, all indices at baseline were comparable between EES and PES. During follow-up, vessel VI and peri-stent plaque VI significantly increased in PES, while they did not change in EES. Delta vessel VI and peri-stent plaque VI were significantly greater in PES compared with EES. Moreover, the lesions whose peri-stent plaque VI increased more than 20% during eight-month follow-up were significantly less frequent in EES compared to PES (EES: 11.5% vs. PES: 41.2%, p=0.007). Late area loss at the stented segment was smaller in EES compared to PES (Figure 3).
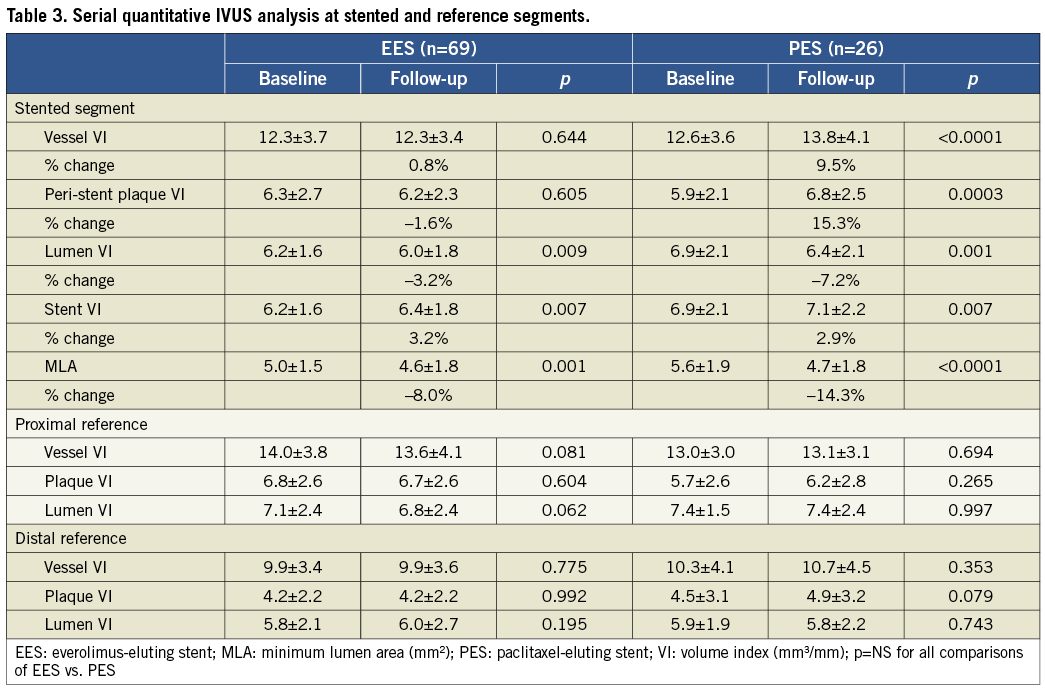
Figure 3. Time course changes of IVUS parameters. Delta vessel VI and peri-stent plaque VI were significantly larger in PES compared with EES during the follow-up period. Delta lumen VI (lumen area loss) at the stented segment was significantly smaller in EES compared with PES. Data are shown as follow-up minus baseline. VI: volume index (mm3/mm); MLA: minimum lumen area (mm2)
In adjacent reference segments, IVUS indices did not show any significant differences between baseline and follow-up or between the two groups except for the slight decrease in lumen VI at proximal adjacent segments in EES.
QUALITATIVE IVUS ANALYSIS
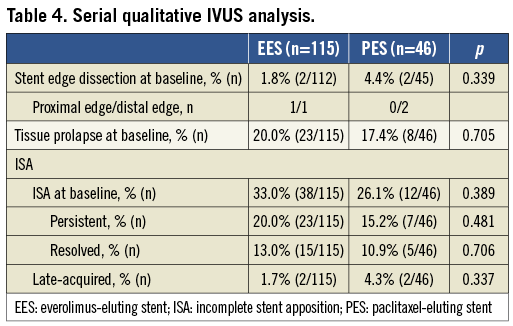
At baseline, the incidence of stent edge dissection and tissue prolapse were not statistically significant between the two groups. Baseline ISA was 33.0% in EES and 26.1% in PES (p=0.389). Late-acquired ISA was infrequent in both groups (Table 4).
CLINICAL OUTCOMES
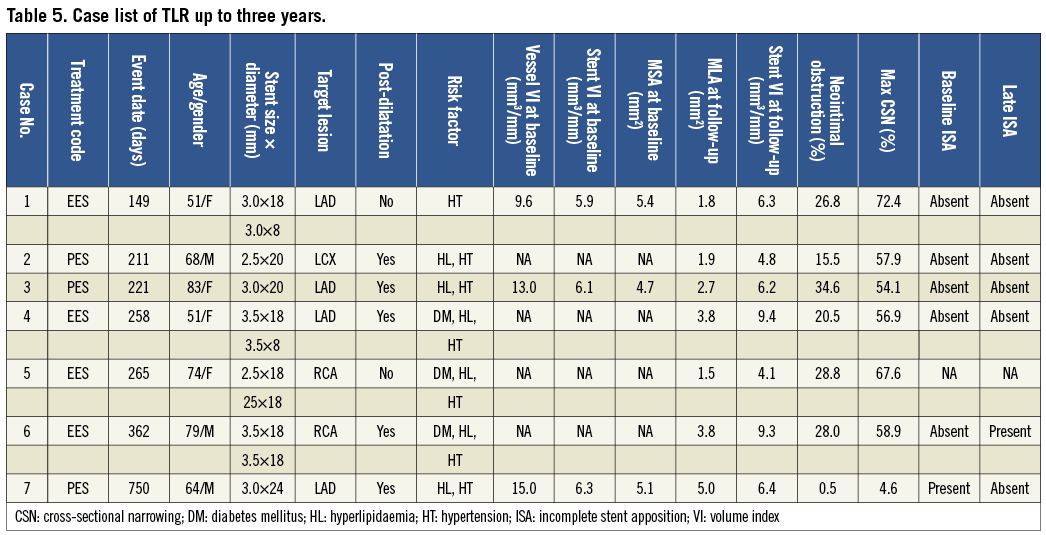
Target lesion revascularisation (TLR) at one year was observed in seven lesions (4.1%) in EES and five lesions (7.0%) in PES (p=0.347). Additional TLR was seen in no lesions in EES and one lesion in PES beyond one year (up to three years), resulting in a cumulative TLR at three years of 4.1% in EES and 8.5% in PES (p=0.178). Table 5 presents summarised cases of TLR for which eight-month IVUS analysis was available. Out of seven follow-up IVUS analyses available, post-dilatation was performed in six lesions of which five lesions had no ISA at baseline, suggesting that these TLR cases achieved adequate stent expansion. One TLR case beyond one year of PES showed minimum neointimal proliferation (% neointimal obstruction: 0.5%) at eight-month follow-up, however, neointimal growth had occurred beyond one year. In patient level analysis, MACE including cardiac death, myocardial infarction or TLR was 6.1% in EES and 9.8% in PES at one year (p=0.339), and 6.1% in EES and 11.5% in PES at three years (p=0.182).
Discussion
The main findings of the detailed IVUS analysis from this prospective, randomised, multicentre, single-blind, controlled trial are as follows: 1) EES significantly reduced neointimal proliferation in stented segments compared with PES at eight-month follow-up; 2) vessel enlargement was seen at stented segments in PES, but not in EES during follow-up; 3) there were no unfavourable edge effects adjacent to either EES or PES; 4) there were no significant differences in the rates of ISA between EES and PES, either at baseline or at eight-month follow-up; and 5) late-acquired ISA was infrequent in both groups.
NEOINTIMAL FORMATION
The % neointimal obstruction of 7.1% in EES observed in this trial was relatively higher than those observed in previous EES trials such as FUTURE I and II (Challenge; Guidant Corp., Santa Clara, CA, USA),6,7 and SPIRIT II8 trials in which the % neointimal obstruction ranged from 2.4 to 2.9%. The longer follow-up period in this present study (eight months) as compared to those in previous trials (six months) may be partially responsible for the relatively higher % neointimal obstruction. Another possibility would be different patient backgrounds and lesion characteristics. Although multiple vessel disease and/or multiple stenting were allowed in this study, previous studies did not include these lesions. It is also important to point out the differences in polymer coating on the stents between the Challenge (bioabsorbable polymer) and the XIENCE V® (durable polymer), and relatively higher drug dose in the Challenge, possibly resulting in different pharmacokinetics and vessel responses.
Compared with PES, EES significantly reduced neointimal proliferation in stented segments at eight-month follow-up. The % neointimal obstruction in PES in this study (11.1%) was consistent with previous pivotal randomised controlled trials using PES.9,10 Combined with previous studies showing 3-5% in the % neointimal obstruction with sirolimus-eluting stents (SES),11,12 the inhibitory effect of neointimal hyperplasia with EES seems to rank between SES and PES. Although the overall amount of neointimal growth was significantly lower in the EES compared with the PES in this IVUS study, the incidence of IVUS estimated restenosis (maximum CSN >60%) was not statistically significant between the two groups, supporting the comparable TLR rate between the two groups in this patient population.
The interpretations of this discrepancy between neointimal proliferation and clinical outcomes are as follows: previous reports suggested the difference of overall neointimal proliferation among currently available drug-eluting stents (DES) in the US may not always directly correspond to clinical outcomes, since in-stent neointima in DES seems to be below the critical threshold for myocardial ischaemia in most patients.3,10,13 In addition, the amount of neointimal hyperplasia in DES may not show a normal distribution curve, showing a markedly skewed shift to the left with variable shapes of the tail ends.5 Therefore, for the evaluation of DES efficacy, it may be insufficient to merely compare the mean value for each DES. The assessment of right tail end might be important to better understand each individual stent’s performance, although this requires considerable patient numbers. In the large scale SPIRIT IV trial,14 EES showed a significant reduction in TLR compared with PES.
PERI-STENT VESSEL RESPONSE
In this IVUS substudy, vessel VI and peri-stent plaque VI at stented segments increased significantly in PES, while they did not show any significant change in EES during follow-up. The vessel response observed in this study was consistent with previous studies.3,10,15 Since SPIRIT II, which compared EES and PES8 did not report detailed peri-stent vessel response, our study is the first report to examine vessel response using patients with the same inclusion/exclusion criteria. In addition, the lesions whose peri-stent plaque VI increased more than 20% during follow-up were significantly more frequent in PES compared with EES. These results suggest there are some interactions between PES and vessel wall components behind the stent while less vessel responses were observed with EES. EES is known to have induced a marked reduction in macrophage content without altering the amount of smooth muscle cells in rabbit atherosclerotic plaques.16 This selective clearance of macrophages occurring by autophagy due to everolimus may cause peri-stent plaque stabilisation after EES implantation. On the other hand, in a rabbit iliac artery model, the extent of inflammation and fibrin deposit was significantly higher in PES compared with bare metal stents.17 Inflammation may cause vessel enlargement, possibly resulting in significant vessel expansion. It is unknown whether vessel enlargement has any clinical impact on the stented segment in the long run, therefore longer-term follow-up after DES implantation is needed.
IMPACT OF DIFFERENT PLATFORMS ON SHORT- AND MID-TERM RESULTS
Platform material and stent design may affect the short-term mechanical properties of metallic stents. Although both EES and PES have an open-cell design, EES is composed of a thin cobalt-chromium alloy whereas PES uses stainless steel struts that are significantly thicker than EES. Despite the differences in stent strut thickness, flexibility of the stent material, and stent delivery systems between EES and PES, there were no statistical differences in the incidence of stent edge dissection, tissue prolapse, and ISA at baseline. These incidences usually depend on patient/lesion characteristics and procedure-related factors including stent/vessel ratio, stent length, maximum balloon pressure, and stent properties. In the current trial, there were no differences in either patient/lesion characteristics or procedural factors between EES and PES. Therefore, there seems to be no apparent difference in basic stent properties affecting immediate outcomes after stent implantation between EES and PES. There was also no significant difference in the frequency of resolved or persistent ISA between EES and PES.
LATE-ACQUIRED INCOMPLETE STENT APPOSITION
Previous studies have shown the incidence of late-acquired ISA to be from 4-13% in SES,4,18,19 from 8-16% in PES,19-21 and 0% in EES.8 The current study showed the incidence of late-acquired ISA was 1.7% in EES and 4.3% in PES, consistent with previous studies. Although there was no significant difference between EES and PES in this study, the low incidence of late-acquired ISA in EES may indicate one aspect related to safety of this new DES technology. In the first generation DES, IVUS features of late-acquired ISA are predominantly due to positive vessel remodelling with stent-vessel detachment.4,20 As previously mentioned, EES did not show vessel enlargement, which may partially contribute to the relatively low incidence of late-acquired ISA after EES implantation.
Limitations
Several limitations exist in the present study. First, follow-up IVUS analysis was limited to a mid-term period at eight-month follow-up. Further studies with longer-term follow-up may be needed, especially to assess late-occurring phenomena. Second, about one third of all lesions were excluded from IVUS analysis due to technical issues during image acquisition such as inconsistent pullback, calibration error and poor image quality. However, patient and lesion characteristics were not significantly different between the entire cohort and the IVUS cohort in this study, therefore selection bias is considered to be minimum. Third, the unbalanced randomisation in a relatively small number of PES patients could lead to some misinterpretation. Moreover, the sample size did not enable adequate statistical power to examine differences of clinical outcomes in this IVUS subgroup analysis. Fourth, frequency of edge dissection and ISA may be affected by the type of imaging modality used for assessment. Finally, there are some intrinsic limitations of IVUS analysis, such as image reconstruction or image interpretation, as previously reported.20
Conclusions
Detailed IVUS analysis from the SPIRIT III trial demonstrated a significantly smaller amount of neointimal hyperplasia in EES compared to PES. Vessel enlargement was seen at stented segments in PES but not in EES during eight-month follow-up. Further trials of EES for the treatment of more complex lesions will be warranted to validate the clinical utility of EES.
Acknowledgements
The authors thank Heidi N. Bonneau, RN, MS, CCA, for her review of this manuscript.
Funding sources
The SPIRIT III trial was supported by Abbott Vascular, Santa Clara, CA, USA.
Conflict of interest statement
K. Sudhir is an employee of Abbott Vascular. G. W. Stone serves on scientific advisory boards for Abbott Vascular/Boston Scientific and received honoraria from Abbott Vascular/Boston Scientific. P. J. Fitzgerald serves as a consultant for Abbott Vascular. All other authors have no conflicts of interest to declare.

