Abstract
Aims: To examine the two year clinical outcomes in dual-vessel disease from the SPIRIT III trial comparing the XIENCE V® everolimus-eluting stent (EES) to the TAXUS Express2™ paclitaxel-eluting stent (PES).
Methods and results: From a total of 1,002 randomised subjects, 103 and 51 patients in the EES and PES groups respectively underwent stenting of two lesions in two vessels (one lesion per epicardial vessel). Two-year event rates were lower in one compared to two-vessel treated patients regardless of stent type. For EES vs. PES, major adverse cardiac events (MACE=cardiac death, MI or TLR) was clinically reduced 35.0% in the single vessel patients (6.5% vs. 9.6%, p=0.09) and was significantly reduced 64% in dual vessel patients (11.9% vs. 30.1%, p=0.006). There was no significant interaction between stent type (EES vs. PES) and the number of stented vessels (two vs. single) for either 2-year TVF and MACE (interaction p values were 0.69 and 0.16, respectively).
Conclusions: In the SPIRIT III randomised trial, patients with both single and dual vessel treatment with EES showed improved clinical outcomes at two years compared to those treated with PES. Follow-up to five years is ongoing.
Introduction
The initial randomised clinical trials (RCTs) comparing the effectiveness of drug eluting stents (DES) to bare metal stents (BMS) were performed in patients with single de novo lesions.1,2 These studies demonstrated substantial reductions in the need for repeat revascularisation with DES compared to BMS. This benefit led to widespread use of DES, although the majority of patients in clinical practice have more complex lesions including multivessel disease, that were not evaluated in the original RCTs of DES vs. BMS. Patients undergoing percutaneous coronary intervention (PCI) with multivessel disease have, in general, worse outcomes than those with single vessel disease.3 Several single and multicentre registries have demonstrated the apparent benefit of DES compared to BMS in “real world” patients undergoing PCI including those patients with multivessel disease.4-6 However, concern about the selection bias makes it difficult to evaluate the outcome of DES compared to BMS in patients with multivessel disease in these observational studies. Moreover, only limited data are available from RCTs comparing outcomes with DES to BMS7,8 or DES to DES9-11 in patients with complex lesions, or multivessel disease. A greater understanding of the relative benefit of DES in patients with multivessel disease would potentially improve decision making regarding stent therapy in patients with complex disease.
The large-scale SPIRIT III randomised controlled clinical trial compared clinical and angiographic outcomes from use of a TAXUS PES to that of a thin-strut XIENCE V EES. The evaluation of both 1-year and 2-year primary outcomes has indicated that the newer generation of DES, the EES, was associated with lower clinical events than the first generation of DES, the PES.10,12 Whether this benefit extended to the subgroup of patients enrolled in the study with two vessel treatment, however, remains uncertain. The goal of this study was to assess clinical outcomes after single vessel vs. double vessel stent treatment following EES and PES treatment.
Methods
Everolimus-eluting stent system
The XIENCE V EES, evaluated in the SPIRIT trials, consists of the coated L-605 Cobalt Chromium (CoCr) alloy MULTI-LINK VISION® (P020047, Japan 21700BZY00468000) or MULTI-LINK MINI VISION (P020047/S003) stent mounted on a MULTI-LINK RX VISION or MULTI-LINK MINI VISION RX delivery system, respectively. All stent diameters were available in 8-28 mm lengths. The coating consisted of two layers: a primer layer and a drug matrix layer. The EES contains everolimus (1 µg/mm2 of everolimus [loaded drug/stent surface area]) which is released from a thin (7.8 µm), non-adhesive, durable, biocompatible fluoropolymer coated onto a low profile (0.0032 in [0.0813 mm] strut thickness), flexible cobalt-chromium stent.
Study population selection and randomisation
The design of the SPIRIT III trial has been previously described.10 In brief, SPIRIT III was a prospective, multicentre, randomised, single-blind, controlled clinical trial in which 1,002 patients with up to two de novo native coronary artery lesions (maximum one lesion per epicardial coronary artery) with a reference vessel diameter (RVD) of 2.5-3.75 mm and lesion length ≤28 mm were randomised in a 2:1 ratio to receive the polymer-based EES (XIENCE V; Abbott Vascular, Santa Clara, CA, USA) or the polymer-based PES (TAXUS Express2; Boston Scientific, Natick, MA, USA).
Patients ≥18 years with stable or unstable angina or inducible ischaemia undergoing PCI were eligible for enrolment. Patients who met the clinical eligibility criteria were invited to participate in this randomised controlled study and were required to provide a signed informed consent prior to enrolment. Key angiographic inclusion criteria included a maximum of two de novo native coronary artery lesions, each in a different epicardial vessel, RVD of ≥2.5 mm and ≤3.75 mm, lesion length ≤28 mm by visual estimation, % diameter stenosis (%DS) of (50% and <100%, TIMI flow of ≥1, non-target vessel percutaneous intervention in non-target vessel planned ≥90 days prior to or >9 months after the index procedure.
Key angiographic exclusion criteria included the aorto-ostial location, left main location, excessive tortuosity, extreme angulation (≥90°), heavy calcification, target vessel containing thrombus, other significant lesions (>40% DS) in the target vessel or side branch for which intervention was required within nine months. If two target lesions were treated, each of these lesions had to meet all angiographic inclusion/exclusion criteria.
Major clinical exclusion criteria included PCI in the target vessel prior to or planned within nine months of the index procedure, or in a non-target vessel within 90 days prior or planned within nine months of index procedure; acute or recent myocardial infarction (MI); left ventricular ejection fraction <30%; use of chronic anticoagulation or immunosuppressive therapy; known autoimmune disease, renal insufficiency, recent major bleed, haemorrhagic diathesis or objection to blood transfusions; contraindications or allergy to any of the study medications, components of the study stents, or iodinated contrast that could not be pre-medicated; elective surgery planned within nine months after the procedure necessitating antiplatelet agent discontinuation; platelet count <100,000 cells/mm3 or >700,000 cells/mm3, white blood cell count <3,000 cells/mm3, serum creatinine >2.5 mg/dL or dialysis, or liver disease; stroke or transient ischaemic attack within six months; co-morbid conditions limiting life expectancy to less than one year or that could affect protocol compliance; and participation in another investigational study that has not yet reached its primary endpoint. The study was approved by the institutional review board at each participating centre, and consecutive, eligible patients signed informed, written consent.
Following confirmation of eligibility, telephone randomisation to EES vs. PES was performed as previously described.10 Subjects were stratified by dual vessel treatment (single vessel vs. double vessel). Although the operators were by necessity un-blinded during the stent implant procedure, the patient and staff involved in follow-up assessments remained blinded until all patients completed the primary endpoint follow-up, with a standardised follow-up interview script used to reduce bias. Patients were considered enrolled in the study from the moment they had been randomised. Protocol specified angiographic follow-up was performed at 240 (±28) days in 436 patients in the randomised study arms.10
Stenting procedure
EES were available in 2.5, 3.0, and 3.5 mm diameters, and in 8, 18, and 28 mm lengths. The full range of US-manufactured PES was available, ranging from 2.5 to 3.5 mm in diameter and from 8 to 32 mm in length. An appropriate length stent was selected sufficient to cover approximately 3 mm of non-diseased tissue on either side of the lesion. In patients receiving multiple stents for a single lesion, 1 to 4 mm of stent overlap was recommended. Post-dilatation was left to the discretion of the investigator. However, if performed, it was only to be done with balloons sized to fit within the boundaries of the stent. If an additional stent was needed to be used for bail out purposes, it was required to be from the same treatment arm as the first implanted stent.
Patients who were not on chronic antiplatelet or aspirin therapy were required to receive aspirin (300 mg pre-procedure and a loading dose of clopidogrel bisulfate (300 mg no later than one hour after the procedure. All patients were to be maintained on 75 mg clopidogrel bisulfate daily for a minimum of six months and (80 mg of aspirin daily throughout the length of the trial (five years) following the index procedure. Subjects who developed hypersensitivity to clopidogrel bisulfate were switched to ticlopidine hydrochloride at a dose in accordance with standard hospital practice.
Clinical follow-up and study endpoints and definitions
Clinical follow-up was scheduled at 30 (±7) days, 180 (±14) days, 240 (±28) days, 270 (±14) days, and 365 (±28) days, with subsequent follow-up yearly (±28 days) through five years. The primary clinical endpoint of the SPIRIT III trial was target vessel failure (TVF), consisting of the composite of cardiac death, MI, or ischaemia-driven target vessel revascularisation (TVR) by either PCI or bypass graft surgery. Secondary endpoints included major adverse cardiac events (MACE), defined as the composite of cardiac death, MI, or ischaemia-driven target lesion revascularisation (TLR), as well as the individual components of TVF and MACE, and stent thrombosis. Stent thrombosis was prospectively defined by protocol as an acute coronary syndrome with angiographic evidence of thrombus within or adjacent to a previously treated target lesion, or in the absence of angiography, any unexplained death or acute MI with ST-segment elevation or new Q-waves in the distribution of the target lesion occurring within 30 days of postprocedure. Definite or probable stent thrombosis was also adjudicated in a post hoc analysis using the Academic Research Consortium (ARC) definitions.13
Statistical analysis for clinical outcomes
A stratified subgroup analysis of angiographic and clinical outcomes, which was pre-specified prior to patient recruitment, was performed for single vessel and double vessel patients to descriptively compare XIENCE V EES and TAXUS PES. Patients who underwent stenting of a single lesion were classified into a single vessel group, whereas patients who received the same DES for both lesions in two treated vessels (one lesion per epicardial vessel) were classified into the two vessel treatment group. There were 103 and 51 patients in the EES and PES groups respectively who underwent stenting of two lesions in two vessels, whereas a single lesion was stented in 566 and 281 patients, respectively.
All randomised patients were included in the analysis of primary and secondary clinical outcomes in the groups to which they were originally allocated to (intention-to-treat principle). Percentages are presented for categorical variables and compared using the Fischer’s exact test. The mean±1 standard deviation (SD) is presented for continuous variables and compared using 2-sided T test. Time-to-event hazard curves were also constructed using Kaplan-Meier estimates and compared by log-rank test. A 2-sided (=0.05 was used for all statistical tests to define significance. All statistical analyses were performed by SAS version 9.1.3 (SAS Institute, Cary, NC, USA).
Results
The baseline patient and lesion characteristics for the EES and PES single and double vessel subgroups are shown in Table 1.
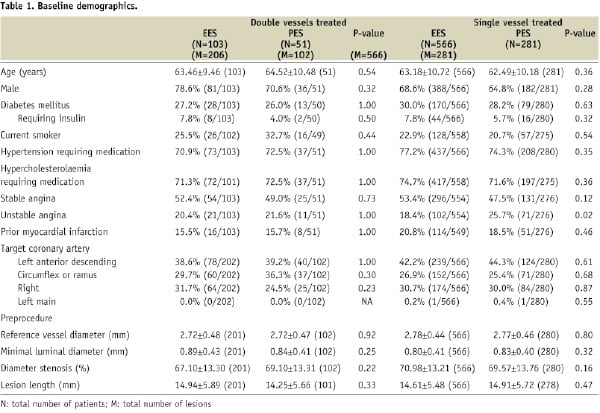
The EES and PES groups overall were well matched, and there were no significant differences in patient and lesion characteristics between the single and double vessel treatment groups. The majority of patients were male, with no difference in gender distribution among the different groups. Diabetes was present in approximately 30% of patients, similarly in all subgroups. The left anterior descending coronary artery (LAD) was a target vessel in approximately 40% of patients in both the single and double vessels subgroups, with similar frequency in the EES and PES groups. The reference vessel diameter and lesion lengths were similar in both the single and double vessel subgroups for both EES and PES groups.
The results of quantitative coronary angiography at 240 days in a subgroup of single and double vessel patients in the EES and PES groups are shown in Table 2.
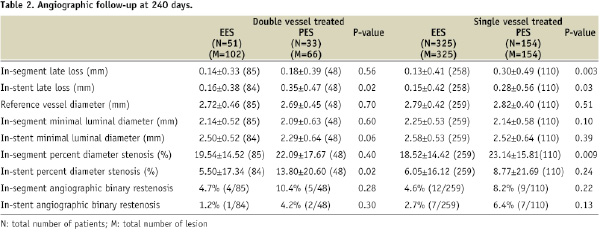
Information was available in 479 single vessel patients: 325 EES and 154 PES; and 84 double vessel patients: 33 EES and 51 PES. In-stent late loss (LL) was lower in both the single and double vessel EES versus PES patients, p=0.03; and p=0.02, respectively. Rates of in-segment binary restenosis were numerically lower for both the single and double vessel EES groups compared to the PES groups, but the p-value did not reach the significant level. Intracoronary ultrasound at 240 days was available in a subgroup of patients at the time of return for angiography (Figure 1).
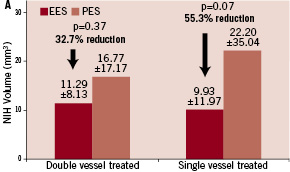
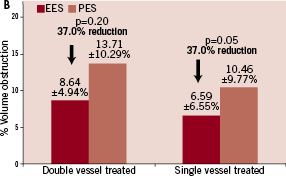
Figure 1. In-stent volumetric measurements of neo-intimal hyperplasia (NIH) volume (mm3) and % volume obstruction (% VO) through 240 days. A. NIH for the double vessel treated subgroups (EES, N=21, M=42; PES, N=13, M=26) and the single vessel treated subgroups (EES, N=139, M=139; PES, N=67, M=67); B. % VO for the double vessel treated subgroups and the single vessel treated subgroups.
The percent volumetric in-stent obstruction was numerically lower in both the single vessel (p=0.05) and double vessel treated (p=0.20) EES groups, compared to the PES groups. Similar lower trends were observed with in-stent neo-intimal hyperplasia.
The 1- and 2-year clinical outcomes for the single and double vessel treated patients are shown in Tables 3 and 4.
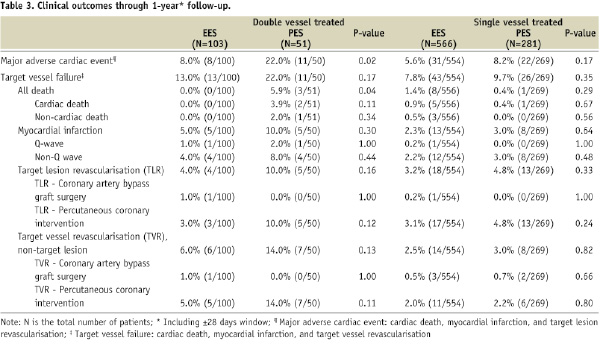
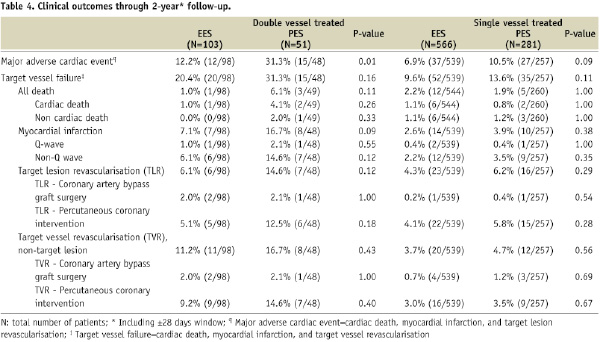
In general, rates of all clinical outcomes were lower for single vs. double vessel treated patients. In the single vessel treated groups, one year rates of adverse clinical events were similar for EES and PES. MACE was numerically lower for EES compared to PES, 5.6% vs. 8.2%, p=0.17. At two years there was a trend for lower MACE with EES, 6.9% compared to PES, 10.5%, p=0.09. Rates of all other clinical outcomes were similar with EES compared to PES. In the double vessel treated groups, MACE was significantly lower with EES compared to PES at one year (8.0% vs. 22.0%, p=0.02) and at two years (12.2% vs. 31.3%, p=0.01). All other adverse events were non-significantly lower for EES compared to PES.
Kaplan-Meier plots of the cumulative incidence of the primary study clinical outcomes through two years for the double vessel treated patients are shown in Figure 2.
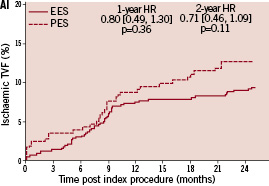
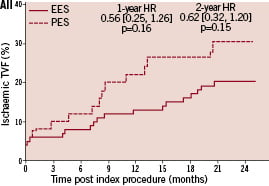
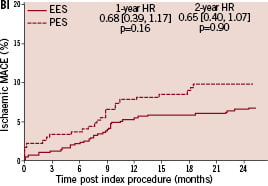
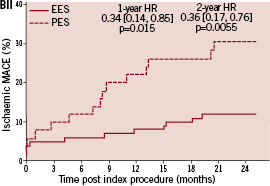
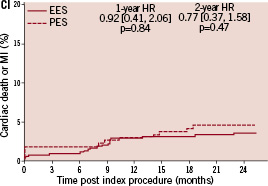
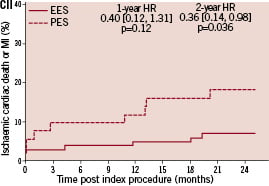
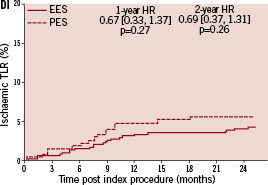
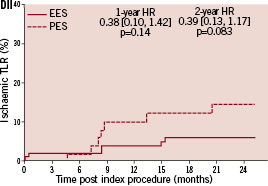
Figure 2. Cumulative incidence rates for the double vessel treated subgroup (EES, N=103; PES, N=51) through two years. AI and AII: Cumulative incidence rates of target vessel failure (TVF) for single and double vessel treated subgroup respectively; BI and II: Cumulative incidence rates of major adverse cardiac events (MACE) for single and double vessel treated subgroup respectively; CI and CII: Cumulative incidence rates of cardiac death or myocardial infarction (MI) for single and double vessel treated subgroup respectively; DI and DII: Cumulative incidence rates of target lesion revascularisation (TLR) for single and double vessel treated subgroup respectively.
The higher absolute rate of clinical events occurred in the first year for all outcomes for both stent types, but continued to increase from year one to two for both EES and PES treated patients. However, the increase in event rates from year one to two was substantially greater for PES treated rather than EES treated patients, resulting in a significant increase in the relative difference in event rates between the two stent types at two years for both single and double vessel treated patients. Kaplan-Meier plots of stent thrombosis per protocol and by ARC definition are shown in Figure 3.
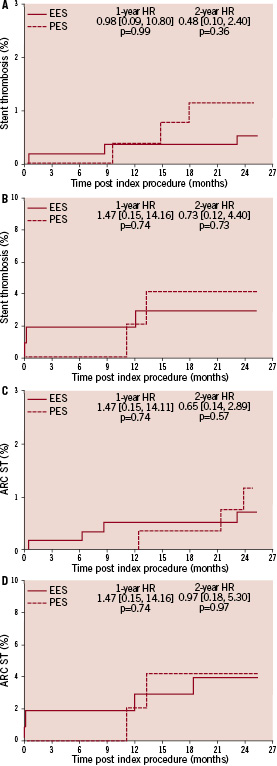
Figure 3. Cumulative incidence rates of stent thrombosis (ST) for the EES and PES patients per protocol and the Academic Research Consortium (ARC) definition in the single and the double vessel treated subgroups through two years. A: Cumulative incidence rates of the protocol defined ST for single vessel treated subgroup; B: Cumulative incidence rates of the protocol defined ST for double vessel treated subgroup; C: Cumulative incidence rates of the ARC defined ST for single vessel treated subgroup; D: Cumulative incidence rates of the ARC defined ST for double vessel treated subgroup.
Rates of stent thrombosis through two years were low for single vessel treated patients in both EES and PES group by either of the criteria. Rates of stent thrombosis by protocol and ARC definitions for two vessel treated patients were similar by either of the criteria, and were overall numerically higher than for single vessel treated patients. EES use vs. PES use resulted in similar rates in stent thrombosis, per protocol definition, in both single vessel treated (0.6% vs. 1.2%, p=0.40) and double vessel treated patients (3.1% vs. 4.3%, p=0.66). Per ARC definition, the rates of stent thrombosis were also comparable in the EES and PES group.
Discussion
This randomised comparison of an EES and a PES demonstrates that adverse clinical outcomes through two years are higher in patients with two vessel disease compared to patients with single vessel disease. Moreover, there was a significant decrease in MACE with EES compared to PES in double vessel treated patients. Importantly, the relative benefit in clinical outcomes after treatment with the EES compared to the PES contributed substantially to the overall clinical benefit observed with the EES12 at two years of clinical follow-up. There are limited data, from randomised trials, regarding clinical outcomes of drug-eluting stent use in complex patients and lesions that are commonly treated in routine clinical practice. The SPIRIT III clinical trial included 1,002 patients with up to two de novo lesions in native coronary arteries, including 154 patients with double vessel disease. These data should provide some reassurance that drug-eluting stent use, particularly EES use, is safe and effective in patients with two vessel disease.
Patients undergoing revascularisation including PCI with multivessel disease have, in general, worse outcomes than those with single vessel disease.3 Although concerns have surfaced about the use of DES in higher risk “off-label” patients and lesions,14 several single and multicentre registries have demonstrated apparent benefit of DES compared to BMS in “real world” patients undergoing PCI including patients with multivessel disease.4-6 However, concern about selection bias makes it difficult to evaluate the outcome of DES compared to BMS in patients with multivessel disease in these observational studies. Few randomised studies evaluating DES outcomes have included patients with complex or multivessel disease.8,9 The TAXUS V and VI studies evaluated outcomes of PES use compared to BMS use in patients with long and complex lesions, but did not include patients with multivessel disease.7,8 In COSTAR II, a randomised comparison of the PES and a novel stent with a bioabsorbable polymer and paclitaxel, 21% of randomised patients had multivessel disease.9 Patients with multivessel disease in that study had numerically higher rates of the primary study outcome, eight months MACE, compared to those with only single vessel disease regardless of stent type, but a formal analysis of this comparison was not performed. In the LEADERS trial11 24.4% of the biolimus-eluting stent treated patients, and 20.7% of the sirolimus-eluting stent treated patients had multivessel disease. Clinical outcomes at one year appeared to be similar for the two stent types with or without multivessel disease. However, no specific analysis of the extent of disease per se on clinical outcomes was performed. In the SPIRIT III study, cumulative clinical event rates were higher in patients with double vessel compared to single vessel disease, consistent with previous studies of both angioplasty alone and BMS use. However, for the first time the findings from this randomised clinical study demonstrate that the use of a new generation DES (in this case EES) are associated with better outcomes than the use of a first generation DES, the PES, after stent treatment of patients with both single and multivessel disease. Consistent with these observations, a larger study, the SPIRIT IV trial (n=3,687),15 recently showed that one year major adverse cardiac events rates were lower in EES compared to PES in both single (3.9% vs. 6.5%) and double vessels (5.2% vs. 10.6%) treated patients.
Quantitative coronary angiographic and ultrasound observations were available from a subgroup of patients at 240 days from the single and double vessel stent treated patients. In-stent and in-segment late loss and measures of percent volumetric obstruction were lower in the EES treated patients than in the PES treated patients for both single and double vessel treated patients. Additionally, late loss and percent volumetric obstruction were similar for both single and double vessel treated patients for both stent types. The late loss observed in the EES group from the SPIRIT III study is similar to that from both SPIRIT FIRST and SPIRIT II, after stent implantation.16,17 Similarly, the late loss observed in the PES patients in the SPIRIT III study are similar to those observed in the TAXUS IV study.18 Since these clinical trials included patients with varying degrees of the extent and severity of coronary artery disease, the findings of similar late loss from both stent types across different study populations provide strong support for the belief that similar results should be expected when applied to higher-risk patients and patients with more complex lesion characteristics than originally studied. In support of this are the one year observations from SPIRIT IV where adverse outcomes were lower in EES compared to PES treated patients in more complex patients and lesion types than has been previously studied in RCT’s.15 Further studies in more complex lesion and patient subgroups will be necessary to confirm these observations.
The clinical outcomes observed in both the overall 1- and 2-year results of the SPIRIT III trial represent the first head-to-head comparison of two drug-eluting stents demonstrating a relative clinical benefit of a newer generation DES (in this case EES) to a first generation DES (in this case PES).10,12 The reasons for the apparent differences are probably multi-factorial. First, as noted above, the EES use resulted in lower in-stent and in-segment late loss at 240 days compared to the PES use in both single and double vessel treated patients. Both animal and clinical studies support the concept that strut thickness is directly proportional to in-stent proliferative responses.19-21 As much as late loss itself predicts the need for repeat revascularisation, it would be expected that target vessel failure would be lower with the thin strut EES compared to the thicker strut PES, as was observed.21 Additional factors may also be important. The EES has a fluoropolymer coating and a thin strut cobalt chromium stent backbone that may be more biocompatible than the proprietary coating used in the PES and a thicker stainless steel stent backbone. Recent animal data suggest that there is less inflammation and a more functional endothelium after the EES placement than the PES placement in a porcine model.22 These observations in conjunction with the findings in this study of increasing relative event rates from year one to two with PES compared to EES, particularly in patients with two vessel treatment, supports the concept that stent biocompatibility may be an important determinant of late DES related events. Continued investigation up to five years will provide important new information concerning this issue.
The observation that MACE was higher for double vessel compared to single vessel treated patients for both stent types merits discussion. The mechanism would appear to be multifactorial as rates of non-STEMI, TLR, and non-TLR TVR were all higher in those with double vessel compared to single vessel treated patients. Further studies will need to be conducted to more precisely define the mechanisms for the difference in outcomes between single and double vessel disease treated patients. It is of concern that the rates of stent thrombosis at two years in the group with double vessel disease are higher than that in the group with single vessel disease independent of stent type. The reasons for the higher rate of stent thrombosis in the patients with double vessel disease compared to single vessel disease are not clear. Several recent studies have evaluated factors that may be associated with late DES thrombosis.23-25 These studies identified early discontinuation of clopidogrel, chronic kidney disease, treatment for STEMI and saphenous vein grafts as important factors associated with late stent thrombosis. Treatment of multivessel disease was not specifically identified as an important risk factor for late DES thrombosis. In the SPIRIT III study, 3/107 patients with discontinuation of clopidogrel before six months postprocedure experienced stent thrombosis, but were equally distributed between the two stent types (2/72 in the EES group vs. 1/35 in the PES group, p=1.00). However, following the discontinuation of clopidogrel between six months to two years, 1/244 EES patients compared to 3/116 PES patients had stent thrombosis (p=0.10). Thus, there is a trend towards lower rates of stent thrombosis in the EES patients compared to the PES patients after discontinuation of clopidogrel. None of the other above mentioned factors identified in these previous studies23-25 were present in the SPIRIT III patient population because the patients with these characteristics were excluded from the study. Although it will be important to confirm this observation in larger studies such as SPIRIT IV, it would seem prudent to recommend longer term dual anti-platelet therapy in patients treated with multivessel DES. Of note, in the COMPARE trial,26 low rates of stent thrombosis were reported in the EES arm (0.7%) vs. the PES arm (2.6%), in a population with over 25% multivessel treated patients.
There are potential limitations of this SPIRIT III substudy that merit discussion. This was a post hoc analysis of 2-year outcomes. As such, reporting and selection biases may have occurred. However, the clinical follow-up was excellent and similar for both stent types. Moreover, the incidence of double vessel treatment was similar for both stent types. Thus, it is unlikely that substantial biases were present in this analysis, although unmeasured biases cannot be excluded. The number of patients with double vessel disease was small. However, previous randomised trials of drug-eluting stents evaluated patients with single vessel disease. Thus, any information from randomised trials evaluating outcomes in patients with more than single vessel disease should be informative. Quantitative coronary angiography and intracoronary ultrasound were not performed at two years. Recent data from the SPIRIT II study at two years indicate that late loss increased from year one to two for the EES, and was similar to that of the PES at two years.27 However, clinical outcomes remained superior with the EES compared to the PES at two years in that study. Although these angiographic findings have not been confirmed, the similarity of the clinical outcomes in SPIRIT II and III suggest that the late loss may not be the most important measure of the EES benefits. The SPIRIT III study was not statistically powered to evaluate clinical outcomes for single and double vessels treated subgroups. Further larger clinical studies such as SPIRIT IV,15 which was powered to evaluate clinical outcomes, will be necessary to confirm that these SPIRIT III observations can be generalised. Finally, this study does not address the relative benefit of DES compared to CABG such as evaluated in ARTS-II and SYNTAX.28,29
Conclusion
This randomised clinical study of an EES and PES demonstrated that clinical outcomes through two years were higher in patients with two vessel compared to single vessel disease, due to higher rates of NSTEMI, TLR, non-TLR TVR, and ST. Moreover, stent type was an important determination of outcomes through two years with a significant decrease in MACE with EES compared to PES in double vessel treated patients.

