Abstract
Aims: Performing percutaneous coronary intervention (PCI) to multiple coronary lesions during the same procedure has potential economic and social advantages. However comprehensive outcome data of real world practice in a large population is limited. We aimed to compare short- and long-term outcomes between patients with multivessel coronary artery disease who either underwent single- or multivessel PCI within the e-SELECT registry.
Methods and results: The e-SELECT registry combines data collected at 320 medical centres in 56 countries where patients received CYPHER Select® or CYPHER Select® Plus sirolimus-eluting stent (SES). Rates of myocardial infarction and major adverse cardiac event (MACE) (defined as any death, myocardial infarction or target lesion revascularisation) were compared between patients undergoing single-vessel versus multivessel PCI. A total of 15,147 patients who satisfied the inclusion criteria were included in the e-SELECT registry. Two thousand two hundred and seventy-eight (2,278) subjects (15%) underwent multivessel PCI and 12,869 (85%) had single-vessel PCI. The mean age was higher in the multivessel PCI group (63 vs. 62 years, p<0.001) and there was a higher prevalence of diabetes mellitus (32.4 vs. 30.0%, p=0.02). Lesions were more complex in the single-PCI group while pre- and post-dilatation were less common in the multivessel PCI group. Myocardial infarction within the first 30 days post PCI was more common in the multivessel PCI group (1.9 vs. 0.8%, p<0.001) and most of the infarctions were periprocedural (1.3 vs. 0.6%, p=0.001). Mortality and myocardial infarction at one-year were higher in the multivessel PCI group resulting in a significantly higher MACE (6.1 vs. 4.6%, p=0.005).
Conclusions: Overall procedural and one year outcomes were excellent for both single- and multivessel procedures. However despite lower lesion complexity, performing multivessel PCI was associated with higher rates of periprocedural myocardial infarction and MACE when compared to single-vessel PCI in the e-SELECT registry.
Introduction
An increasing number of patients are treated with percutaneous coronary intervention (PCI) and a recent analysis of revascularisation procedures from the United States demonstrated a substantial decrease of coronary artery bypass grafting (CABG) procedures compared to PCI1. The use of drug-eluting stents (DES) has dramatically reduced the rate of in-stent restenosis (ISR)2 and as a consequence percutaneous coronary intervention can be performed in two or three arteries and in increasingly long and complex atheromatous lesions.
Performing multivessel PCI in a single sitting has potential economic and social advantages, and most of the times the cumulative duration of dual antiplatelet therapy (especially after implantation of DES) can be shortened. Additionally, re-gaining arterial access for staged PCI is associated with a small but significant risk of access site injury and bleeding3,4.
In a recent report from the HORIZONS-AMI trial, multivessel PCI of the culprit lesion and bystander disease was associated with a higher mortality and stent thrombosis5. However, comprehensive outcome data of real world practice in a large population is limited.
Using data from the e-SELECT registry we aimed to compare outcomes in patients undergoing single vs. multivessel PCI. Outcomes were compared with particular reference to potential differences in both procedural and one-year clinical outcomes.
Methods
Details of the e-SELECT registry have recently been published6. Briefly, e-SELECT was conducted at 320 medical centres (listed in an on-line supplement) in 56 countries where sirolimus-eluting stents (SES) have been approved for commercial use. Baseline data were collected between May 2006 and April 2008 in consecutive and eligible patients who underwent successful implantation of ≥1 CYPHER Select® or CYPHER Select® Plus (Cordis Corporation, Bridgewater, NJ, USA) SES according to standard clinical practice and procedural techniques.
The protocol specified very few inclusion or exclusion criteria. Lesions could be pre-treated with any technique or device, such as balloon angioplasty, cutting balloon or atherectomy, but implantation of SES in each target lesion during the index procedure was mandatory. All postoperative medical management, including antithrombotic therapy, was prescribed according to usual local practices. The protocol was approved by the ethics committee of each participating medical centre and the patients granted their consent to participate in the registry. Patients for whom the collection of dependable follow-up information was unlikely and those who received a stent other than a CYPHER SES during the index procedure were excluded.
Data collection and management
The data collected by the e-SELECT registry include demographic information, cardiovascular history, comorbidity, lesion and procedure characteristics, and antithrombotic regimens. The patients were followed at 30, 180 and 360 days by telephone communication, office visit, or by contacts with primary physicians or referring cardiologists.
The data were collected electronically at each participating medical centre and transferred to an independent data management organisation (KIKA Medical, Nancy, France). After verification of their consistency, the data were analysed by an independent clinical research organisation (Cardialysis, Rotterdam, The Netherlands). The accuracy of data collection was monitored by an independent organisation (Covance, Princeton, NJ, USA) in 20% of the overall sample, at 100 centres selected by a stratification scheme based on patient enrolment, region of the world and rate of data outliers. The consistency and accuracy of data contained in the source documentation versus that entered in the electronic database were verified, using an anonymous procedure to preserve confidentiality. The data were consistent when present in both the source documents and in the electronic database, and accurate when the electronic database fully matched the data entered in the source documents. Using these definitions, overall data consistency was 99%; the accuracy of baseline data was 96%, and that of adverse events recorded during follow-up was 93.2%.
Endpoints of the e-SELECT registry
The primary endpoint of the registry was a composite of definite and probable ST at one-year follow-up, as defined by the Academic Research Consortium (ARC7). The secondary endpoints at one-year included: cardiac and non-cardiac death, myocardial infarction (MI), and MACE (defined as any death, MI or target lesion revascularisation).
Statistical analysis
For all patients, standard descriptive statistics were used for baseline, lesion, and procedural characteristics and for clinical results. Continuous variables are presented as mean±standard deviations, medians and range, and categorical variables such as numbers and percentages. Cumulative rates of adverse clinical events were calculated with event-specific adjusted denominators, so that all patients experiencing an event within 360 days or followed up for at least 330 days after the index procedure contributed to the denominator. No missing value imputation was performed. All statistical analyses were performed with SAS, version 9.1 or higher software (SAS Institute, Cary, NC, USA).
Results
A total of 15,147 patients satisfying the inclusion criteria were included in the e-SELECT registry. Baseline clinical characteristics of patients with multivessel and single-vessel PCI are presented in Table 1. Two thousand two hundred and seventy-eight (2,278) subjects (15%) underwent multivessel PCI and 12,869 (85%) had single-vessel PCI. In the multivessel PCI group, the mean age was higher (63 vs. 62 years, p<0.001), there was a higher prevalence of diabetes mellitus (32.4 vs. 30.0%, p=0.02), and the reported prevalence of multivessel disease was much higher (98% vs. 36%, p<0.001). The single-vessel PCI group had a significantly higher prevalence of prior PCI and CABG. The Charlson Comorbidity Index Score and the EuroSCORE (standard and logistic) were similar between the two groups (Table 1).
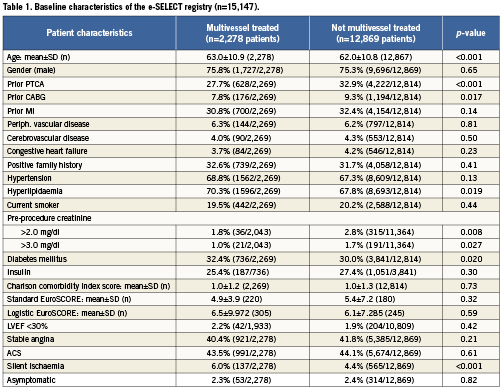
Target vessel and lesion characteristics
Details regarding the target vessel and lesion characteristics are summarised in Table 2. The patients in the multivessel PCI group were more likely to undergo treatment of a native artery (99.2 vs. 97.7%, p<0.001) and the target lesion was less likely to be in-stent restenosis (8.0 vs. 13.1%, p<0.001). The subjects in the single-vessel group had more complex lesions reflected by the higher proportion of chronic total occlusions, prevalence of visible thrombus and higher rates of B2 and C lesions according to the American Heart Association (AHA) classification (Table 2).
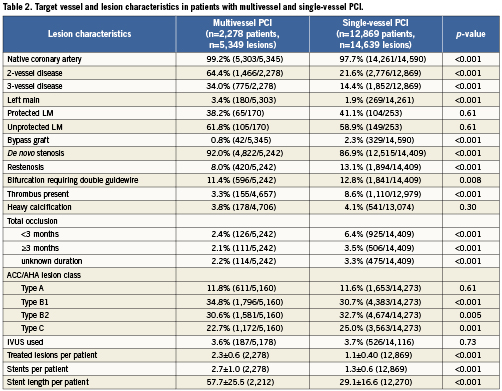
Procedural characteristics
Pre-procedural treatment with aspirin, clopidogrel or ticlopidine was more common in the multivessel PCI group than in single-vessel PCI (90.5 vs. 88.9, p=0.03). The rate of pre-treatment with dual antiplatelet therapy was similar between both groups (57.7 vs. 59%, p=0.28). Figure 1 demonstrates the ratios of pre- and post-dilatation in the study population. Patients in the multivessel PCI group were less likely to undergo predilatation (58.4 vs. 66.5%, p<0.001) and also less likely to undergo post-dilatation after stent implantation (32.3 vs. 37.5%, p<0.001). Intravascular ultrasound (IVUS) use was similar between both groups (3.6 vs. 3.7%, p=0.73). Total stent length per patient was markedly longer in the multivessel group (57.7 vs. 29.1 mm, p<0.001).
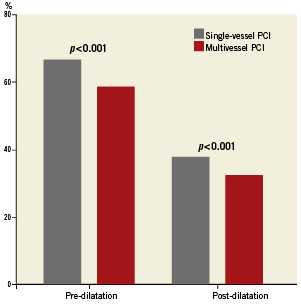
Figure 1. Ratios of pre- and post-dilatation in patients with single- and multivessel PCI.
Staged procedures were planned in 6.4% of patients in the multivessel and in 6.5% of the single-vessel PCI patients (p=0.93).
Principal effectiveness and safety results
30-day outcomes Table 3 and Figure 2 summarise the principal outcomes at 30-days and one-year. There was no significant difference in the incidence of mortality, cardiac mortality and stent thrombosis rates between the multivessel and single-vessel PCI group at 30-days. The incidence of myocardial infarction was higher in the multivessel PCI group (1.9 vs. 0.8%, p<0.001) and most of the infarctions were non-Q-wave MI and related to the procedure (1.3 vs. 0.6%, p=0.001). The incidence of target vessel revascularisation (TVR) was similar between the groups but patients in the multivessel PCI group had a higher rate of target lesion revascularisation (TLR; 0.7 vs. 0.3%, p=0.027) at 30-days.
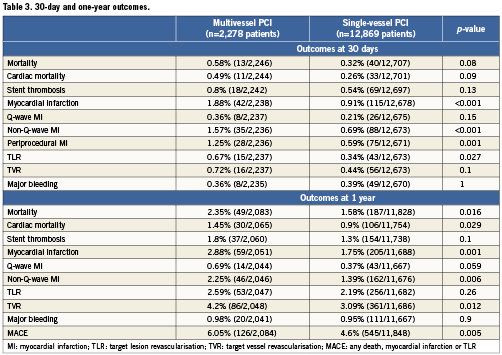
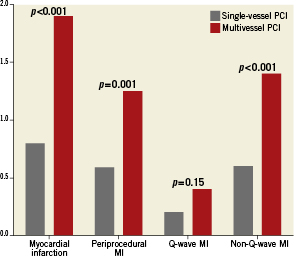
Figure 2. The 30-day outcomes in patients undergoing single-vessel and multivessel PCI.
360-day outcomes Both overall and cardiac mortality were higher in the multivessel PCI group at one-year (Table 3). Most of the infarctions were non-Q-wave and related to the procedure (Figure 3). The rate of Q-wave infarctions was not different between the two groups and there was no significant difference in the TLR, TVR or major bleeding between both groups.
In-hospital MACE (1.6 vs. 0.8%, p<0.001) and one-year MACE (6.1 vs. 4.6%, p=0.005) was higher in patients with multivessel PCI. Rates of stent thrombosis were higher in the multivessel PCI group but the difference did not reach statistical significance.
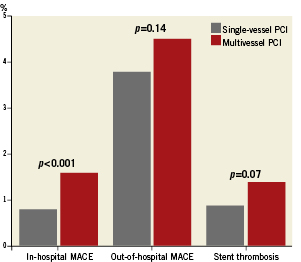
Figure 3. The 360-day outcomes in patients undergoing single-vessel and multivessel PCI.
Discussion
This analysis of the e-SELECT registry demonstrates that despite individual lesions having lower complexity, patients undergoing multivessel PCI, who were older and had more diffuse coronary artery disease (CAD), had higher rates of adverse events. These patients had twice as many lesions treated as well as total stented length and number of stents implanted. The in-hospital MACE rate was higher and this was mainly driven by the incidence of periprocedural MI. At one-year, overall and cardiac mortality and incidence of myocardial infarction were all significantly higher in the multivessel PCI group. There was also a trend towards a higher incidence of stent thrombosis in the multivessel PCI group, possibly explained by the doubling of the total stent length in this group.
In the e-SELECT registry there was a significant difference in the rates of lesion preparation and post-dilatation between the single-vessel and multivessel disease groups. Consequently this must reflect an increased rate of direct stenting by operators when multiple lesions were treated. Although direct stenting is effective in selected lesions8,9 this strategy might not be optimal when lesions are more complex and/or calcified. In the e-SELECT population only 11.8% of the lesions were Type A and almost one quarter of them were Type C lesions according to the AHA classification10. It is possible to speculate that operators were tempted to minimise multivessel procedure times by direct stenting at least one target lesion.
Although post-dilation with minimally compliant balloons was considered routine during the early coronary stent experience, improved stent and balloon technology has led to considerable variation in post-dilation usage in routine contemporary practice. Despite these improvements in stent technology, incomplete stent expansion remains a predictor of both stent thrombosis and later in-stent restenosis11,12. Adjunctive balloon post-dilatation does not distort stent anatomy13 and has the potential to reduce adverse outcomes by optimising expansion of drug-eluting stents14. In the e-SELECT registry there was a significant difference in the rates of post-dilatation between the single-vessel and multivessel disease groups. This observation that pre- and post-dilatation were used less commonly in the multivessel PCI group could be an important factor in the observed increased one-year MACE rates.
In the e-SELECT registry, patients in the multivessel PCI group had a higher proportion of myocardial infarctions at 30-days and most of these were non-Q-wave infarctions related to the procedure. In-hospital MACE, defined as the composite endpoint of death, myocardial infarction and target-lesion revascularisation, was higher in the multivessel PCI group.
Periprocedural myocardial infarction is an important prognostic marker following PCI15,16. Distal embolisation and side branch occlusion are known to be contributing factors and the risk of myocardial injury during PCI increases with lesion complexity reflected by the SYNTAX score15. One might assume that patients with more atheroma might be at higher procedural risk and this might in itself explain the higher incidence of procedural infarction. However this explanation does not appear to be applicable, as the multivessel group had less complex lesions (according to the AHA classification10) and less high-risk clinical features17 (higher rates of prior CABG, severe renal failure, bypass grafts and in-stent restenosis). Alternatively one could assume that when treating longer lengths of atheroma in the same sitting, enzyme release will increase in a cumulative fashion. This explanation appears to be more plausible, bringing to question the rationale for systematically treating multiple non-complex lesions during the same procedure when it may not always be clinically indicated. Notably, recruitment to e-SELECT predated the publication of the FAME study18 and it is possible that increased use of fractional flow reserve measurement might have resulted in less stent implantation, reduced periprocedural injury, and might potentially have been associated with greater clinical benefit.
Limitations
It is important to mention some limitations of this study. These are registry data of selected patients who underwent a successful procedure with only SES at index.
There was probably a selection bias as it can be assumed that the second or third vessel in the multivessel procedures were only approached after the first lesion was successfully treated. This is supported by the fact that there were more complex lesions in the single-vessel group.
Patients undergoing multivessel PCI more often had multivessel disease than the single-vessel PCI, somewhat limiting the comparison between the two groups.
The patients with acute ST-elevation myocardial infarction most often only received treatment of the culprit lesion: they would more often have been in the single-vessel group, and were known to have a higher ST risk. Additionally there were more saphenous vein grafts (SVG) treated in the single-vessel group.
No recommendations were made for biomarker measurements post-procedure: periprocedural MI were most probably underestimated in both groups.
Conclusions
In this registry, multivessel PCI was associated with higher rates of periprocedural myocardial infarction when compared to single-vessel PCI. Additionally, rates of overall death, cardiac death and non-Q-wave infarctions were higher in the multivessel PCI group. Patients treated in the multivessel PCI group had lower rates of pre- and post-dilatation and this could have contributed to the increased rate of sub-optimal outcomes. The results of our study suggest that multivessel PCI in a single sitting should be the exception rather than the rule in patients needing treatment in more than one coronary artery.
Conflict of interest statement
Dr. Urban has served as consultant for Cordis and Biosensors. Dr. Banning has received research funding from Boston Scientific and Cordis; and his salary is partially funded by the NIHR Oxford Biomedical Research Centre. Dr. Bartorelli has served as a consultant to Abbott Vascular and is on the Speakers’ Bureaus of Bracco and Cordis, Johnson & Johnson. DrBaux has been a full-time employee of and has stock options in Cordis, Johnson & Johnson. Dr. Džavik has served as consultant for and received a research grant from Abbott Vascular and has received educational funds from Cordis. Dr. Legrand has served as a consultant for Cordis, Johnson & Johnson, and is a member of the scientific advisory board of Abbott. Dr. Nyakern has been a salaried consultant for Cordis, Johnson & Johnson. Dr. Spaulding has received research funding from Cordis, Abbott, Stentys, and Lilly; has received speaker fees from Cordis, Lilly, and Pfizer; was on the scientific advisory board of Cordis; and has been a full-time employee of Cordis, Johnson & Johnson since July 1, 2010.
Online data supplement

