Abstract
Aims: We sought to investigate medium-term outcome of percutaneous coronary intervention (PCI) compared with coronary artery bypass grafting (CABG) in patients with triple-vessel coronary artery disease (TVD).
Methods and results: We identified 2,981 patients with TVD (PCI: N=1,825, CABG: N=1,156) among 15,939 patients with first coronary revascularisation enrolled in the CREDO-Kyoto PCI/CABG registry cohort-2. Excess adjusted three-year risk of the PCI group relative to the CABG group for death/myocardial infarction (MI)/stroke was significant (HR 1.47 [95% CI: 1.13-1.92, p=0.004]). Adjusted risk for all-cause death was also significantly higher with PCI as compared with CABG (HR 1.62 [95% CI: 1.16-2.27, p=0.005]), while risk for cardiac death was neutral between the two groups (HR 1.3 [95% CI: 0.81-2.07, p=0.28]). PCI was also associated with a markedly higher risk for any coronary revascularisation. Regarding the analysis stratified by the SYNTAX score, the adjusted HR of PCI relative to CABG for death/MI/stroke was 1.66 (95% CI: 1.04-2.65, p=0.03) in the low-score (<23: N=874, and N=257), 1.24 (95% CI: 0.83-1.85, p=0.29) in the intermediate-score (23-32: N=638, and N=388), and 1.59 (95% CI: 0.998-2.54, p=0.051) in the high-score (≥33: N=280, and N=375) tertiles, respectively.
Conclusions: PCI as compared with CABG was associated with significantly higher risk for serious adverse events in TVD patients.
Introduction
Percutaneous coronary intervention (PCI) has been performed with increasing frequency in patients with severe coronary artery disease such as left main coronary artery (LMCA) disease or triple-vessel coronary artery disease (TVD) since the introduction of drug-eluting stents (DES)1,2. However, medium-term clinical outcomes of PCI relative to coronary artery bypass grafting (CABG) in patients with severe coronary artery disease have not yet been adequately evaluated. The SYNTAX (SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery) randomised trial is the first dedicated trial comparing PCI with CABG in these high-risk categories of patients3. Three-year results from the SYNTAX trial suggested that excess risk of PCI relative to CABG for all-cause mortality as well as a composite of death, myocardial infarction (MI) and stroke was significant in the TVD subset, but not in the LMCA disease subset4. Furthermore, in the SYNTAX trial, medium-term risks of PCI relative to CABG for serious cardiovascular events were successfully stratified by the SYNTAX score tertiles, suggesting that PCI as compared with CABG was associated with poorer outcomes with increasing coronary anatomic complexity. One of the major limitations of these subgroup analyses in the SYNTAX trial, however, was the apparent lack of satisfactory statistical power in evaluating serious cardiovascular events not including repeat revascularisation.
Therefore, we intended to evaluate medium-term clinical outcome of PCI as compared with CABG in a greater number of patients with TVD from a large observational database in Japan. In an attempt to overcome the limitations regarding the issue of comparability regarding coronary anatomy between the PCI and CABG groups in an observational study, coronary anatomic complexities were assessed by utilising the SYNTAX score5.
Methods
STUDY POPULATION
The CREDO-Kyoto (Coronary REvascularization Demonstrating Outcome Study in Kyoto) PCI/CABG registry cohort-2 is a physician-initiated non-company-sponsored multicentre registry enrolling consecutive patients undergoing first coronary revascularisation among 26 centres in Japan between January 2005 and December 2007. The relevant ethics committees in all 26 participating centres (Online Appendix A) approved the research protocol. Because of retrospective enrolment, written informed consents from the patients were waived; however, we excluded those patients who refused participation in the study when contacted for follow-up.
The study design and patient enrolment of the registry have been previously described in detail2. Among 15,939 patients enrolled in the registry, the study population for the current pre-specified analysis of the CREDO-Kyoto PCI/CABG registry cohort-2 consisted of 2,981 patients with TVD (PCI: N=1,825, and N=1,156), excluding those patients with refusal for study participation, concomitant non-coronary surgery, acute myocardial infarction presentation, single or double-vessel disease, and LMCA disease (Online Figure 1).
DATA COLLECTION FOR BASELINE CHARACTERISTICS AND SYNTAX SCORE
Demographic, angiographic and procedural data were collected from hospital charts according to pre-specified definitions by the experienced research coordinators in the independent research organisation (Research Institute for Production Development, Kyoto, Japan) (Online Appendix B). Patients with TVD were identified by the angiographic information recorded in the hospital charts. Definitions for clinical characteristics are described in Online Appendix C.
The SYNTAX score was calculated by using the SYNTAX score calculator (available at http://www.syntaxscore.com) in the dedicated SYNTAX score committee (Online Appendix D). All analyses were conducted blinded to the clinical data. Intra- and inter-observer variabilities for the calculation of the SYNTAX score in our group have been previously reported6. The cut-off values for the SYNTAX score tertiles (low-score: <23, intermediate-score: 23-32, and high-score: ≥33) were defined according to the analysis in the SYNTAX trial3,4.
ENDPOINTS
The primary outcome measure was defined as a composite of all-cause death, MI, and stroke. Other pre-specified endpoints included all-cause death, cardiac death, non-cardiac death, MI, stroke, and coronary revascularisation. Death was regarded as cardiac in origin unless obvious non-cardiac causes could be identified. MI was defined according to the definition in the Arterial Revascularization Therapy Study7. Stroke was defined as ischaemic or haemorrhagic stroke either occurring during the index hospitalisation or requiring hospitalisation with symptoms lasting >24 hours. Coronary revascularisation was defined as either PCI or CABG for any reasons. Clinically-driven coronary revascularisation was defined as those procedures driven by ischaemic symptoms or objective evidences of myocardial ischaemia or both. Scheduled staged coronary revascularisation procedures performed within three months of the initial procedure were not regarded as follow-up events, but were included in the index procedure.
DATA COLLECTION FOR FOLLOW-UP EVENTS
Collection of follow-up information was mainly conducted through review of hospital charts by the clinical research coordinators in the independent research organisation. Additional follow-up information was collected through contact with patients, relatives and/or referring physicians by sending mails with questions regarding vital status, additional hospitalisations, and status of antiplatelet therapy. Death, MI, stent thrombosis (ST) and stroke were adjudicated by the clinical events committee (Online Appendix E).
STATISTICAL ANALYSIS
Categorical variables are presented as numbers and percentages and are compared with the chi-square test. Continuous variables are expressed as mean value±SD or median with interquartile range (IQR), and are compared using the Student’s t-test or Wilcoxon rank-sum test based on their distributions.
Cumulative incidence was estimated by the Kaplan-Meier method and differences were assessed with the log-rank test. The effects of PCI relative to CABG for individual endpoints were expressed as hazard ratios (HR) and their 95% confidence intervals (CI). We estimated the HR by Cox proportional hazard models adjusting for 30 clinically relevant factors listed in Table 1. Continuous variables were dichotomised by clinically meaningful reference values or median values. Proportional hazard assumptions for potential independent risk-adjusting variables were assessed on the plots of log (time) versus log (-log [survival]) stratified by the variable, and the assumptions were verified to be acceptable for all the variables. We incorporated the 26 participating centres in the Cox proportional hazard models as the stratification variable.
Because the issues of selection biases and unmeasured confounders are inherent limitations of observational studies, a propensity score matching analysis was conducted as a sensitivity analysis (Online Appendix F). Furthermore, in our previous report comparing PCI with CABG in the bare metal stent era, we discussed the issues of selection bias and unmeasured confounders in observational studies, suggesting that it would be appropriate to exclude elderly patients when attempting observational comparisons between CABG and PCI considering the potential presence of profound patient selection bias in the elderly population8. Therefore, we conducted an analysis stratified by 75 years of age as an additional sensitivity analysis.
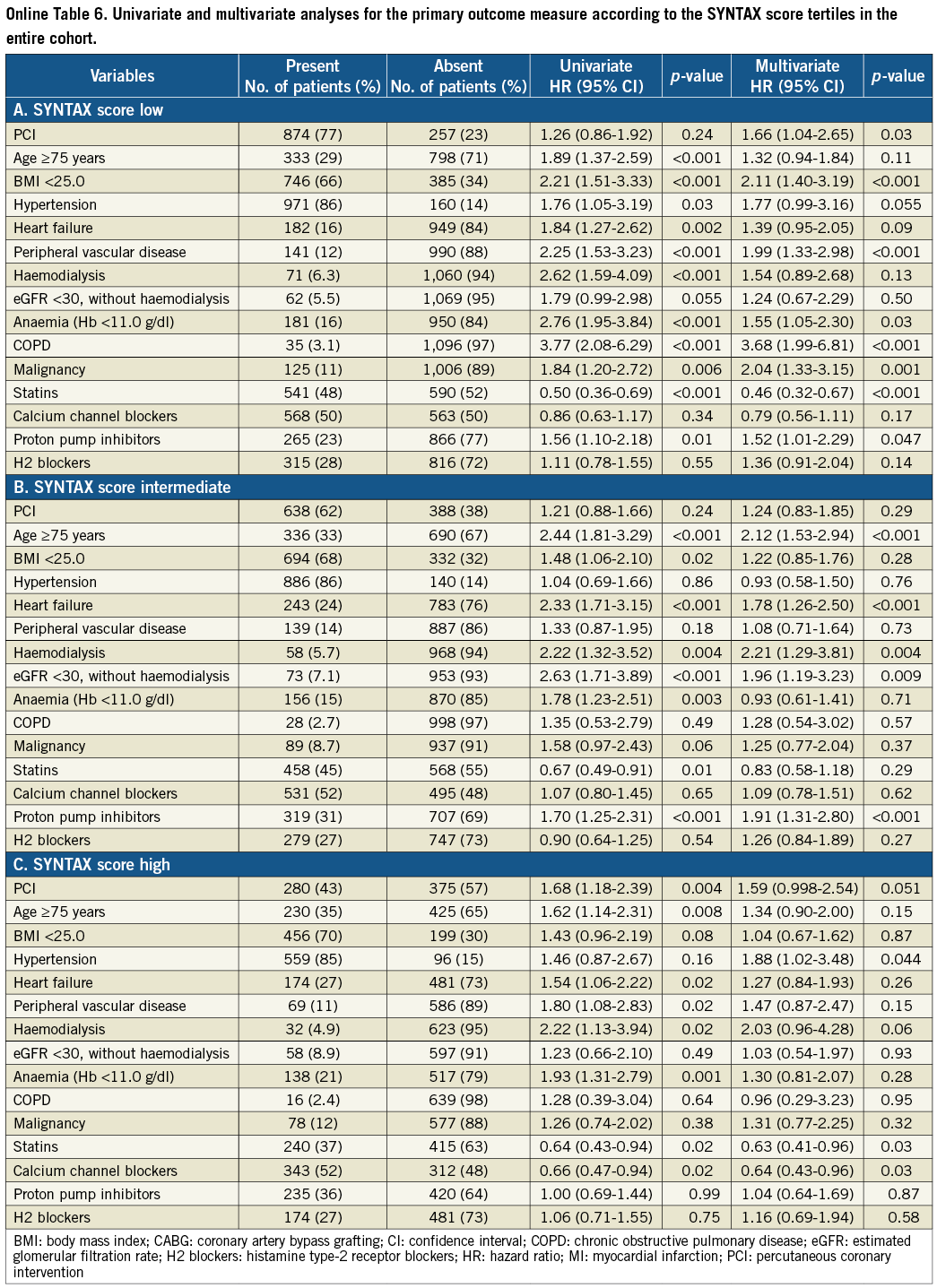
As a subgroup analysis, unadjusted and adjusted risks of PCI relative to CABG for clinical events were evaluated in each SYNTAX score tertile. In addition to the variable of PCI against CABG, we included 14 variables with a p-value <0.05 in the full model described previously.
Statistical analyses were conducted by two physicians (J. Tazaki and H. Shiomi) and a statistician (T. Morimoto) with the use of JMP 8.0 (SAS Institute Inc., Cary, NC, USA) software and SAS 9.2 (SAS Institute Inc., Cary, NC, USA). All the statistical analyses were two-tailed and p-values <0.05 were considered statistically significant.
Results
BASELINE CHARACTERISTICS
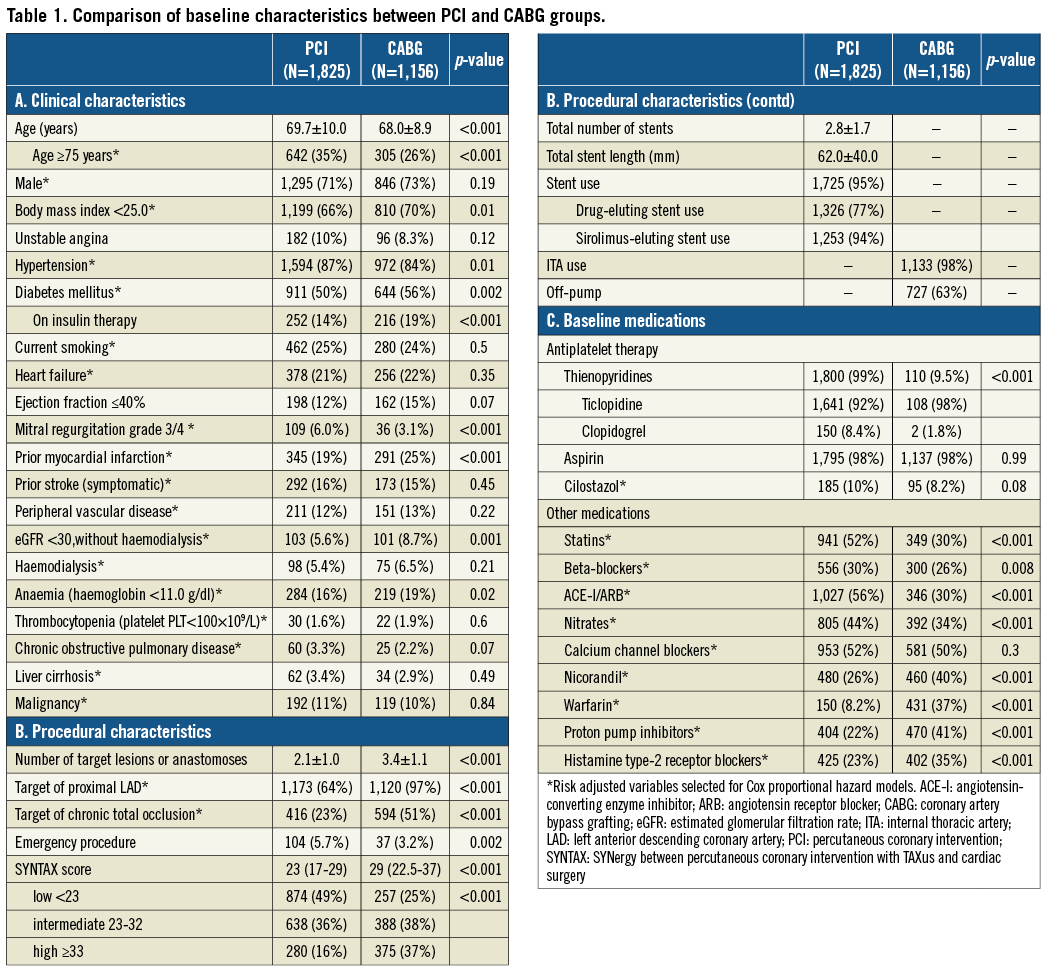
Baseline clinical characteristics were significantly different between the PCI and CABG groups (Table 1). Patients in the PCI group were older, and more often had hypertension and severe mitral regurgitation, while patients in the CABG group more often had smaller body mass index, diabetes, prior MI, renal failure, and anaemia.
The CABG group included more patients with complex coronary anatomy and who had a greater number of target lesions or anastomoses (Table 1). The SYNTAX scores were available in 2,812 patients (94%). The median SYNTAX score was significantly greater in the CABG group than in the PCI group (29 [IQR 22.5-37] versus 23 [IQR 17-29], p<0.001). In the PCI group, stents were used in 95% of patients and at least one DES was used in 77% of patients. Sirolimus-eluting stents (SES) were used in the majority of DES patients (94%). In the CABG group, at least one internal thoracic artery was used in 98% of patients, and the prevalence of off-pump CABG was high (63%). Baseline medications were also significantly different between the two groups (Table 1).
CLINICAL OUTCOME IN THE ENTIRE STUDY POPULATION
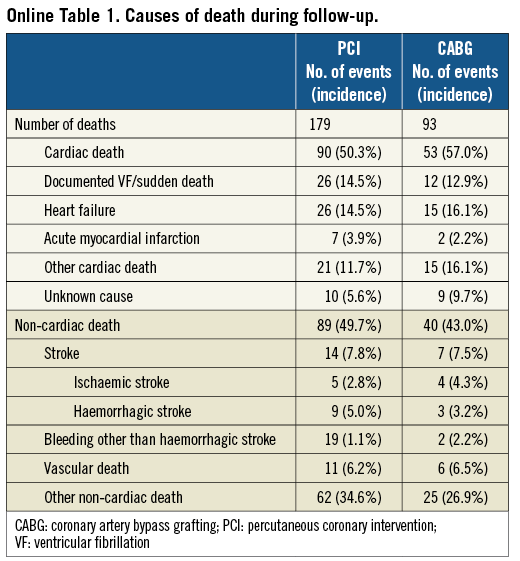
The cumulative three-year incidence of the primary outcome measure was significantly higher in the PCI group than in the CABG group (18.3% vs. 15.2%, log rank p=0.03) (Figure 1). After adjusting confounders, PCI as compared with CABG remained associated with a significantly higher risk for the primary outcome measure in the entire study population (HR 1.47 [95% CI: 1.13-1.92, p=0.004]) (Table 2). Although PCI was also associated with a significantly higher risk for all-cause death, the risk for cardiac death was similar between the two groups (Table 2, Figure 1). Distributions of causes of death were remarkably similar between the two groups (Online Table 1).
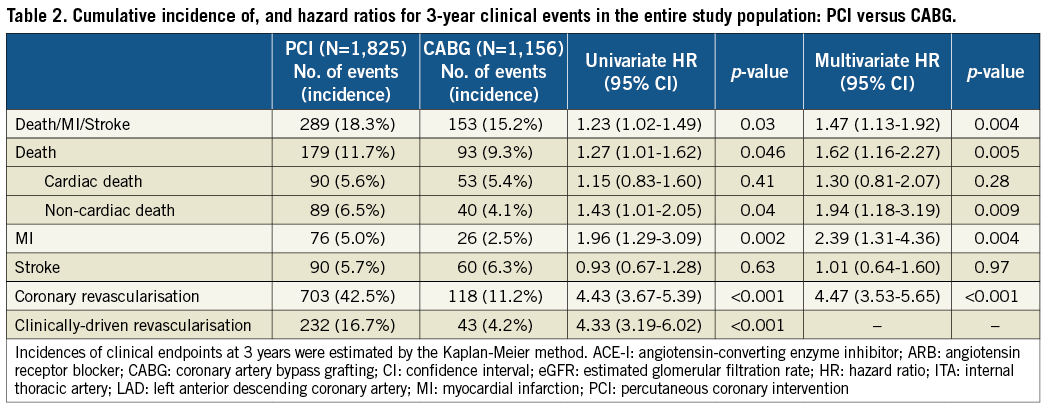
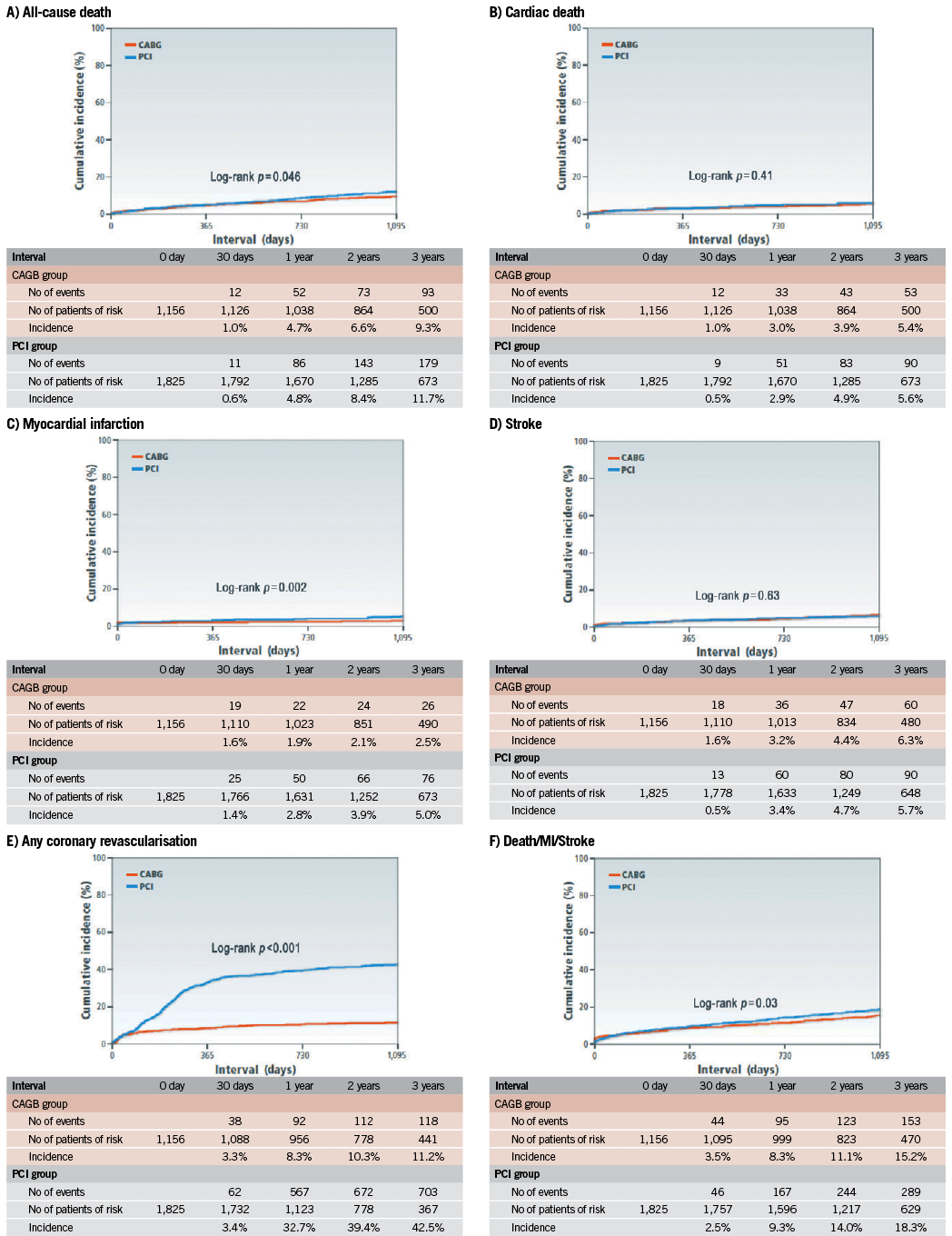
Figure 1. Kaplan-Meier event curves: PCI versus CABG for: A) all-cause death; B) cardiac death; C) myocardial infarction; D) stroke; E) any coronary revascularisation; and F) a composite of all-cause death, myocardial infarction and stroke.CABG: coronary artery bypass grafting; PCI: percutaneous coronary intervention
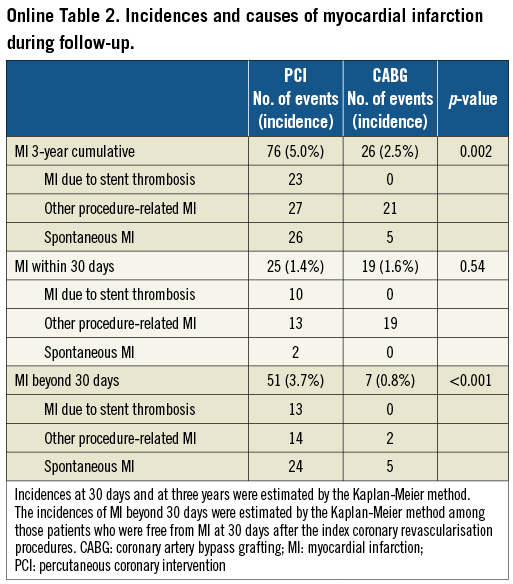
PCI as compared with CABG was associated with a significantly higher unadjusted and adjusted risk for MI (Table 2, Figure 1), although the prevalence of death due to acute myocardial infarction was similarly low in both PCI and CABG groups (Online Table 1). The higher MI risk of PCI relative to CABG was due to excess of spontaneous MI, MI related to ST, and MI related to repeat procedure beyond 30 days after the index procedure (Online Table 2). Cumulative three-year incidence of definite ST in the PCI group was low (1.3%).
The risk for stroke was not different between the two groups. PCI as compared with CABG was associated with a markedly higher risk for any coronary revascularisation as well as clinically-driven coronary revascularisation (Table 2, Figure 1).
SENSITIVITY ANALYSES
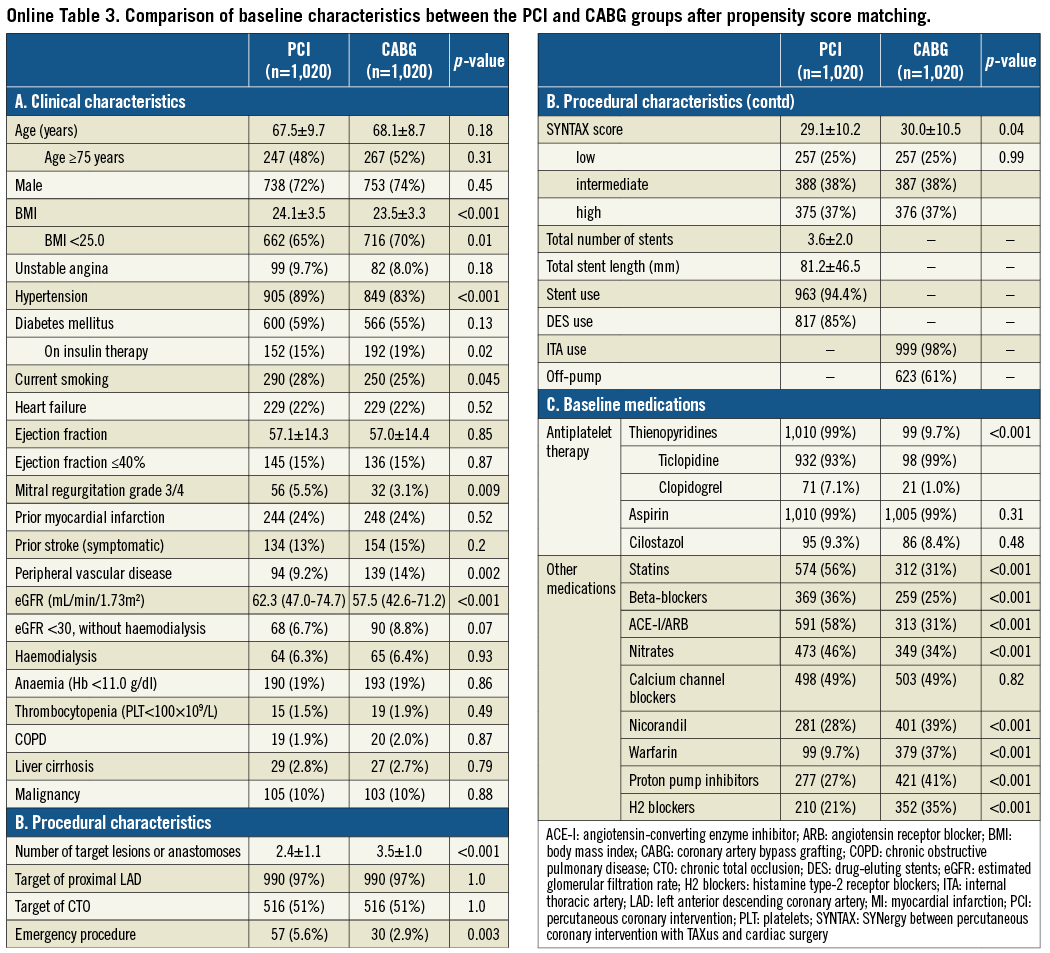
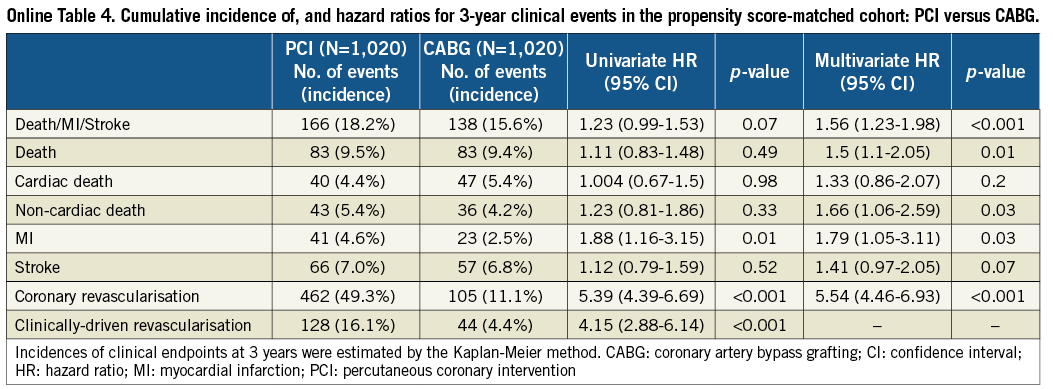
After propensity score matching as a sensitivity analysis, baseline characteristics of the PCI and CABG groups were much more comparable than those in the entire study population (Online Table 3). Results from the propensity score matching analyses were fully consistent with those results derived from the Cox proportional hazard models in the entire cohort (Online Table 4, Online Figure 2).
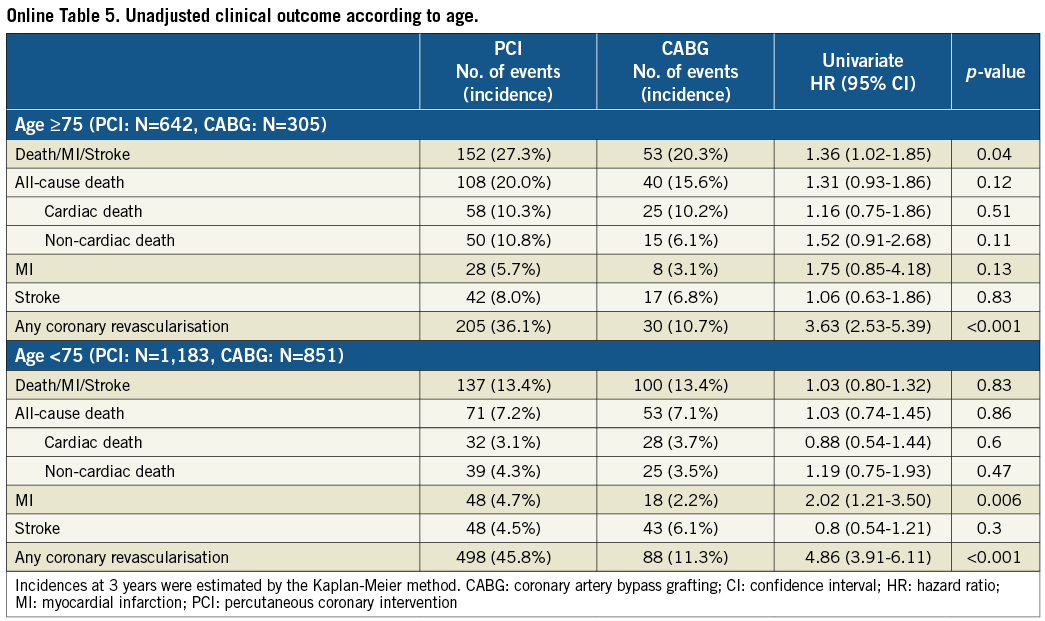
In patients ≥75 years of age (PCI: N=642, CABG: N=305), cumulative incidence of the primary outcome measure was higher in the PCI group than in the CABG group (27.3% vs. 20.3%, log-rank p=0.04), while it was not different between the two groups in patients <75 years of age (PCI: N=1,183, CABG: N=851) (13.4% vs. 13.4%, log rank p=0.83) (Online Table 5). Cumulative incidence of all-cause death as well as non-cardiac death was similar between the two groups in patients <75 years of age (7.2% vs. 7.1%, log rank p=0.86, and 4.3% vs. 3.5%, log rank p=0.47, respectively), suggesting less impact of unmeasured confounders in this age population.
SYNTAX SCORE AND CLINICAL OUTCOME
Clinical outcome was compared between the PCI and CABG groups among the three categories of the SYNTAX score. Cumulative incidence of the primary outcome measure was not different between the PCI and CABG groups in patients with low and intermediate SYNTAX score, while in patients with high SYNTAX score it was markedly higher in the PCI group than in the CABG group (Figure 2). In patients with a high SYNTAX score, the incidences of all-cause death and cardiac death were also higher in the PCI group than in the CABG group. However, after adjusting confounders, the HR of PCI relative to CABG for the primary outcome measure was 1.66 (95% CI: 1.04-2.65, p=0.03) in the low-score, 1.24 (95% CI: 0.83-1.85, p=0.29) in the intermediate-score, and 1.59 (95% CI: 0.998-2.54, p=0.051) in the high-score tertile, respectively (Online Table 6).
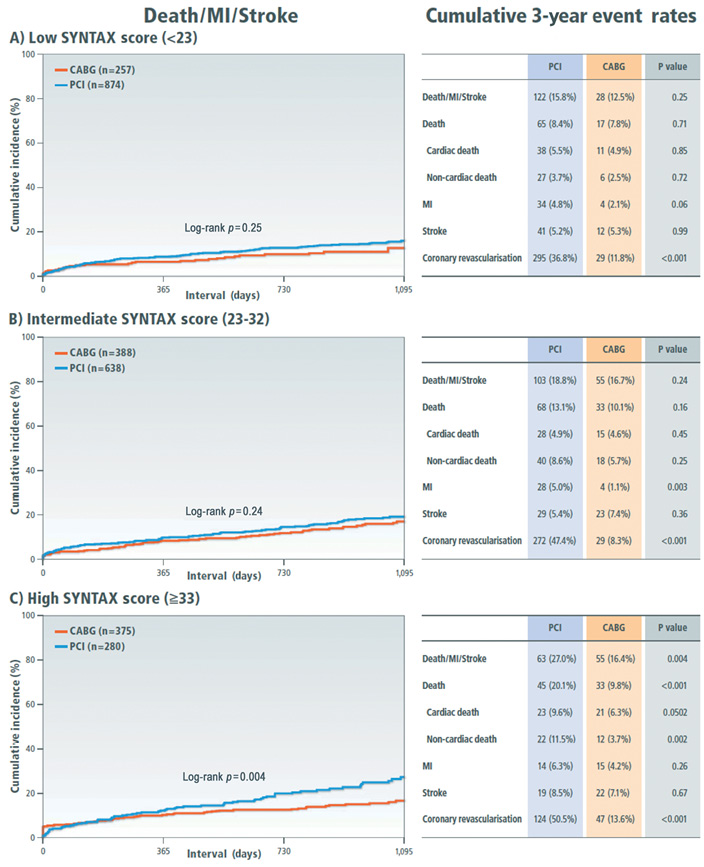
Figure 2. Kaplan-Meier event curves comparing PCI with CABG for a composite of all-cause death, myocardial infarction and stroke stratified by the SYNTAX score tertiles. A) Low SYNTAX score category (<23); B) intermediate SYNTAX score category (23-32); C) high SYNTAX score category (≥33). CABG: coronary artery bypass grafting; MI: myocardial infarction; PCI: percutaneous coronary intervention
Discussion
The main findings in this study were as follows: 1) consistent with the SYNTAX randomised trial results, PCI as compared with CABG was associated with significantly higher risk for serious cardiovascular events in patients with TVD; 2) in contradiction to the SYNTAX randomised trial results, the benefit of using the SYNTAX score for risk stratification could not be clearly demonstrated.
The SYNTAX randomised trial is the first dedicated trial comparing PCI using paclitaxel-eluting stents (PES) with CABG in high-risk patients such as TVD and LMCA disease. Three-year results from the SYNTAX trial suggested that the incidences of all-cause death as well as MI in the TVD stratum were significantly higher after PCI than after CABG4. However, since the SYNTAX trial was not powered for evaluating all-cause death or MI, this observation should be regarded as hypothesis-generating. Furthermore, PES has been proven to be inferior to SES with higher rates of stent thrombosis and repeat revascularisation9. The strength of the current study was the sample size (N=2,981), larger than the SYNTAX randomised trial (N=1,095), and the inclusion of consecutive patients with TVD undergoing first coronary revascularisation reflecting the real-world clinical practice. In DES patients, SES rather than PES was predominantly used in the current study.
Results from previous observational studies comparing PCI using DES with CABG in TVD patients were conflicting. A report from the New York Cardiac Registry suggested that risk for death and MI was significantly higher after PCI with DES than after CABG in patients with TVD10. The Asan Medical Center-Multivessel Revascularisation Registry reported that there was no difference in the five-year incidence of death, MI, or stroke between PCI and CABG11. A relatively small number of patients with TVD, and a very small number of patients with high SYNTAX score were enrolled in this registry. The findings in the current observational study, enrolling the largest ever number of TVD patients with SYNTAX score assessment, were in line with those in the SYNTAX trial as well as those in the recently reported FREEDOM trial12, favouring CABG in terms of lower risk for a composite of death, MI, and stroke. The lower risk for MI after CABG was most remarkable. Patients with TVD were generally associated with extensive coronary atherosclerosis and were treated using a greater number of stents. Therefore, the risk for stent-related MI, MI related to repeat procedure, and spontaneous MI related to atherosclerotic non-target lesions was higher after PCI than after CABG, which bypassed not only the critical lesions but also proximal non-critical atherosclerotic lesions. Furthermore, the degree of coronary revascularisation was generally significantly more complete in the CABG group than in the PCI group. Less complete revascularisation in the PCI group might be one of the reasons for higher serious cardiovascular event risk. Regarding risk for stroke, the risk for stroke through the entire follow-up period was not different between the two groups, although there was a trend for a higher stroke rate in the CABG group at 30 days.
The risk for all-cause mortality in the current study was higher after PCI than after CABG, which was also consistent with the SYNTAX trial result. Although the sample size was much greater in the current study, the observational study design severely hampered drawing conclusions on survival benefit of CABG over PCI in TVD. The higher mortality rate in the PCI group was driven by the excess of non-cardiac death, while the risk for cardiac death was similar between PCI and CABG. Furthermore, consistent with our previous observation, survival outcome of patients <75 years of age, constituting the majority of the patient population, was similar between PCI and CABG8. In a recent report on 101 patients undergoing non-emergent LMCA PCI, surgical ineligibility dictating treatment selection was common, and advanced age was the leading reason cited for CABG ineligibility12. Therefore, it seems to be highly likely that a large proportion of patients ≥75 years of age in the current study actually represented those who were treated with PCI due to CABG ineligibility. Surgical ineligibility was reported to be independently associated with worse long-term outcomes after adjusting for standard risk scores13. Considering potential selection bias and unmeasured confounders, we should be careful in drawing conclusions on survival outcome after PCI and CABG from the current study.
The risk for coronary revascularisation was markedly higher after PCI than after CABG, particularly within the first year after the index procedures. Although the majority of the coronary revascularisation procedures were non-clinically driven procedures due to the high prevalence of scheduled follow-up angiography by local site protocols, the risk for clinically driven coronary revascularisation was also higher after PCI than after CABG. Despite the introduction of DES, the incidence of repeat coronary revascularisation after initial PCI was very high in the TVD population.
In the SYNTAX trial, the incidence of a composite of all-cause death, MI, or stroke in the PCI group as compared with the CABG group was higher in the SYNTAX score high and intermediate tertiles, but not in the SYNTAX score low tertile. However, the benefit of using SYNTAX score for risk stratification could not be clearly demonstrated in the current study. Both the SYNTAX randomised trial and the current study were underpowered for this subgroup analysis. In the FREEDOM trial12, there was no interaction between the SYNTAX score subgroups and risk of PCI relative to CABG, although the analysis was also underpowered. In this context, it is intriguing that total stent length was not an independent predictor of mortality in the SYNTAX trial14. Given the limitations related to the subgroup analysis, further investigations should be mandatory to establish an ideal risk stratification model according to coronary anatomic complexities in choosing PCI or CABG in TVD patients.
Limitations
There are several important limitations in this study. First and most importantly, the observational study design precluded drawing definitive conclusions regarding superiority of either PCI or CABG due to selection bias and unmeasured confounders. Surgical ineligibility was reported to occur on the basis of risk factors not captured by the American College of Cardiology-National Cardiovascular Data Registry (ACC-NCDR)13. Although the CREDO-Kyoto PCI/CABG registry cohort-2 evaluated potential risk factors more extensively than the ACC-NCDR, we failed to incorporate many important risk factors such as frailty, cognitive dysfunction, active malignancy, and systemic infection, etc. However, the conclusion of the current study suggesting superiority of CABG in terms of serious adverse events seemed to be robust, because multiple sensitivity analyses demonstrated consistent findings. Second, the duration of follow-up was not long enough to evaluate long-term outcome of coronary revascularisation. Very late stent thrombosis of SES has been reported to occur constantly at a rate of 0.3% per year without attenuation at least up to five years after initial stent implantation15. Third, SYNTAX score data were not available in all patients. Fourth, the subgroup analysis stratified by the SYNTAX score was confounded by imbalances in baseline clinical characteristics and was obviously underpowered to evaluate the primary outcome measure, although the number of patients with TVD enrolled in the current study was greater than that in the SYNTAX trial. Also, regarding the relation between SYNTAX score tertiles and clinical outcome, multivariate analyses in the subgroup analyses should be interpreted carefully due to the relatively small number of events in each subgroup. Finally, because patient demographics, practice pattern, and clinical outcome in patients undergoing PCI and CABG in Japan are markedly different from those outside Japan1,2,8, care should be taken in extrapolating the current study results outside Japan.
Conclusions
Consistent with the observation in the SYNTAX randomised trial, the current observational study demonstrated that PCI as compared with CABG was associated with significantly higher risk for serious adverse events in patients with TVD.
Funding
This study was supported by the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Online Appendix A. List of the participating centres and the investigators for the CREDO-Kyoto PCI/CABG registry cohort-2
CARDIOLOGY
Kyoto University Hospital: Takeshi Kimura; Kishiwada City Hospital: Mitsuo Matsuda, Hirokazu Mitsuoka; Tenri Hospital: Yoshihisa Nakagawa; Hyogo Prefectural Amagasaki Hospital: Hisayoshi Fujiwara, Yoshiki Takatsu, Ryoji Taniguchi; Kitano Hospital: Ryuji Nohara; Koto Memorial Hospital: Tomoyuki Murakami, Teruki Takeda; Kokura Memorial Hospital: Masakiyo Nobuyoshi, Masashi Iwabuchi; Maizuru Kyosai Hospital: Ryozo Tatami; Nara Hospital, Kinki University Faculty of Medicine: Manabu Shirotani; Kobe City Medical Center General Hospital: Toru Kita, Yutaka Furukawa, Natsuhiko Ehara; Nishi-Kobe Medical Center: Hiroshi Kato, Hiroshi Eizawa; Kansai Denryoku Hospital: Katsuhisa Ishii; Osaka Red Cross Hospital: Masaru Tanaka; University of Fukui Hospital: Jong-Dae Lee, Akira Nakano; Shizuoka City Shizuoka Hospital: Akinori Takizawa; Hamamatsu Rosai Hospital: Masaaki Takahashi; Shiga University of Medical Science Hospital: Minoru Horie, Hiroyuki Takashima; Japanese Red Cross Wakayama Medical Center: Takashi Tamura; Shimabara Hospital: Mamoru Takahashi; Kagoshima University Medical and Dental Hospital: Chuwa Tei, Shuichi Hamasaki; Shizuoka General Hospital: Hirofumi Kambara, Osamu Doi, Satoshi Kaburagi; Kurashiki Central Hospital: Kazuaki Mitsudo, Kazushige Kadota; Mitsubishi Kyoto Hospital: Shinji Miki, Tetsu Mizoguchi; Kumamoto University Hospital: Hisao Ogawa, Seigo Sugiyama; Shimada Municipal Hospital: Ryuichi Hattori, Takeshi Aoyama, Makoto Araki; Juntendo University Shizuoka Hospital: Satoru Suwa
CARDIOVASCULAR SURGERY
Kyoto University Hospital: Ryuzo Sakata, Tadashi Ikeda, Akira Marui; Kishiwada City Hospital: Masahiko Onoe; Tenri Hospital: Kazuo Yamanaka; Hyogo Prefectural Amagasaki Hospital: Keiichi Fujiwara, Nobuhisa Ohno; Kokura Memorial Hospital: Michiya Hanyu; Maizuru Kyosai Hospital: Tsutomu Matsushita; Nara Hospital, Kinki University Faculty of Medicine: Noboru Nishiwaki, Yuichi Yoshida; Kobe City Medical Center General Hospital: Yukikatsu Okada, Michihiro Nasu; Osaka Red Cross Hospital: Shogo Nakayama; University of Fukui Hospital: Kuniyoshi Tanaka, Takaaki Koshiji, Koichi Morioka; Shizuoka City Shizuoka Hospital: Mitsuomi Shimamoto, Fumio Yamazaki; Hamamatsu Rosai Hospital: Junichiro Nishizawa; Japanese Red Cross Wakayama Medical Center: Masaki Aota; Shimabara Hospital: Takafumi Tabata; Kagoshima University Medical and Dental Hospital: Yutaka Imoto, Hiroyuki Yamamoto; Shizuoka General Hospital: Katsuhiko Matsuda, Masafumi Nara; Kurashiki Central Hospital: Tatsuhiko Komiya; Mitsubishi Kyoto Hospital: Hiroyuki Nakajima; Kumamoto University Hospital: Michio Kawasuji, Syuji Moriyama; Juntendo University Shizuoka Hospital: Keiichi Tanbara
Online Appendix B. List of the clinical research coordinators
RESEARCH INSTITUTE FOR PRODUCTION DEVELOPMENT
Kumiko Kitagawa, Misato Yamauchi, Naoko Okamoto, Yumika Fujino, Saori Tezuka, Asuka Saeki, Miya Hanazawa, Yuki Sato, Chikako Hibi, Hitomi Sasae, Emi Takinami, Yuriko Uchida, Yuko Yamamoto, Satoko Nishida, Mai Yoshimoto, Sachiko Maeda, Izumi Miki, Saeko Minematsu
Online Appendix C. Definitions of clinical characteristics
Baseline clinical characteristics, such as prior myocardial infarction, heart failure, hypertension, current smoking, atrial fibrillation, chronic obstructive lung disease, liver cirrhosis and malignancy were regarded as present when these diagnoses were recorded in the hospital charts. Elderly patients were defined as those patients ≥75 years of age. Unstable angina was defined as Braunwald classification type 3. Diabetes was defined as treatment with oral hypoglycaemic agents and/or insulin, prior clinical diagnosis of diabetes, glycated haemoglobin level ≥6.5%, or blood glucose level ≥200 mg/dl. Blood glucose test results in the acute phase of acute myocardial infarction were not used for the diagnosis of diabetes. Prior stroke included both ischaemic and haemorrhagic stroke and was defined as stroke with neurological symptoms lasting >24 hours. Peripheral vascular disease was regarded as being present when carotid, aortic, or other peripheral vascular disease was being treated or scheduled for surgical or endovascular interventions. Left ventricular ejection fraction (LVEF) was measured either by contrast left ventriculography or echocardiography. Patients with LVEF ≤40% were regarded as having left ventricular dysfunction. Renal function was expressed as estimated glomerular filtration rate (eGFR) calculated by the Modification of Diet in Renal Disease (MDRD) formula modified for Japanese patients1. Anaemia was defined as blood haemoglobin level less than 11.0 g/dl. Thrombocytopenia was defined as platelet count <100×109/L. A bifurcation lesion was defined as a lesion requiring insertion of a guidewire into the side branch. Baseline medications were regarded as present if prescribed during the index hospitalisation.
Online Appendix D. List of the SYNTAX score committee members
Masao Imai (Kyoto University Hospital), Kyohei Yamaji (Kokura Memorial Hospital), Kazuya Nagao (Osaka Red Cross Hospital), Shunsuke Funakoshi (Kyoto University Hospital), Natsuhiko Ehara (Kobe City Medical Center General Hospital), Koji Hanazawa (Tenri Hospital), Akihiro Tokushige (Kagoshima University Hospital), Tomohisa Tada (Deutsches Herzzentrum), Masahiro Natsuaki (Kyoto University Hospital), Junichi Tazaki (Kyoto University Hospital), Hiroki Shiomi (Kyoto University Hospital), Yoshihiro Kato (Saiseikai Noe Hospital), Mamoru Hayano (Gunma Cardiovascular Center), Syunichiro Niki (Hirakata Kohsai Hospital), Nobuya Higashitani (Hamamatsu Rosai Hospital), Mitsuhiko Yahata (Kyoto University Hospital), Sayaka Saijo (Kyoto University Hospital), Yuichi Kawase (Japanese Red Cross Wakayama Medical Center).
Online Appendix E. List of the clinical event committee members
Mitsuru Abe (Kyoto Medical Center), Hiroki Shiomi (Kyoto University Hospital), Tomohisa Tada (Deutsches Herzzentrum), Junichi Tazaki (Kyoto University Hospital), Yoshihiro Kato (Saiseikai Noe Hospital), Mamoru Hayano (Gunma Cardiovascular Center), Akihiro Tokushige (Kagoshima University Hospital), Masahiro Natsuaki (Kyoto University Hospital), Tetsu Nakajima (Kyoto University Hospital).
Online Appendix F. Propensity score matching analysis
We computed the propensity score by using logistic regression analysis with the dependent variable being the mode of coronary revascularisation (PCI or CABG), and the 14 independent variables potentially influencing the choice of mode of coronary revascularisation (age ≥75, diabetes, heart failure, stroke, eGFR <30 without haemodialysis, haemodialysis, anaemia, thrombocytopenia, chronic obstructive pulmonary disease, liver cirrhosis, malignancy, target of proximal left anterior descending coronary artery, target of chronic total occlusion, and SYNTAX score tertile). Using only the propensity score, patients in the CABG group were matched to PCI patients using a greedy matching strategy where patients are initially matched by five decimal places of the propensity score followed by decreasing numbers of decimal places2. This resulted in 1,020 patients with CABG matched to 1,020 patients with PCI. Clinical outcomes were compared between the PCI and CABG groups in these propensity score-matched cohorts. Cumulative incidence was estimated by the Kaplan-Meier method and differences were assessed with the log-rank test. Adjusted comparisons were also conducted by using multivariate Cox proportional hazard models with those independent variables which could potentially influence clinical outcomes but which were not included in the calculation of the propensity score (body mass index <25.0, hypertension, use of statins, use of calcium channel blockers, use of proton pump inhibitors, and use of histamine type 2 receptor blockers).
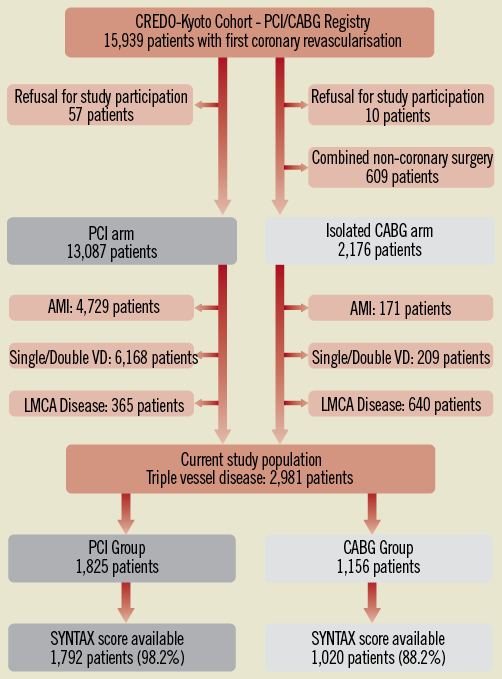
Online Figure 1. Study flow chart. AMI: acute myocardial infarction; CABG: coronary artery bypass grafting; LMCA: left main coronary artery; PCI: percutaneous coronary intervention; SYNTAX: SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery; VD: vessel disease
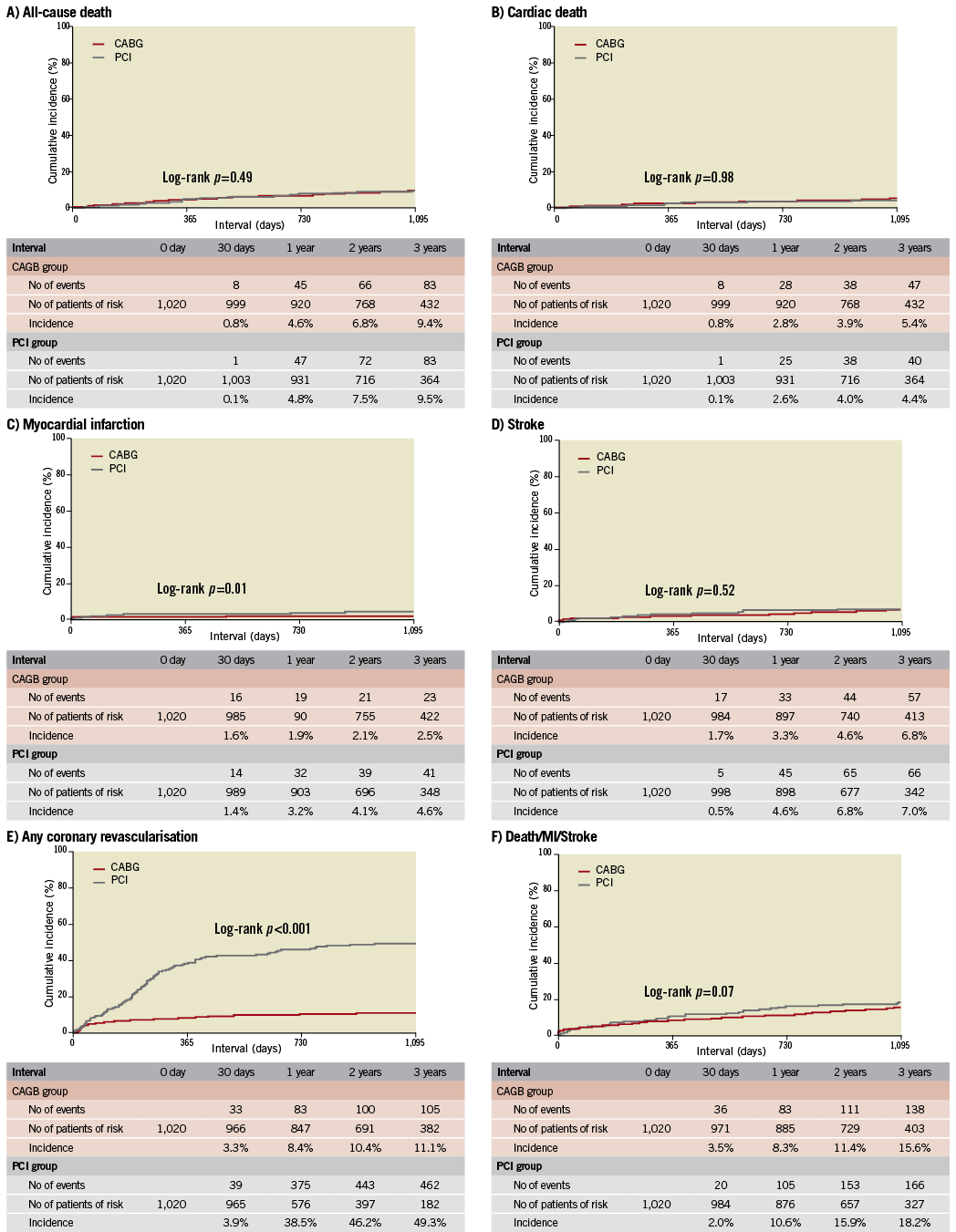
Online Figure 2. Kaplan-Meier event curves in the propensity score-matched cohort: PCI versus CABG. (A) for all-cause death, (B) for cardiac death, (C) for myocardial infarction, (D) for stroke, (E) for any coronary revascularisation, and (F) for a composite of all-cause death, myocardial infarction and stroke. CABG: coronary artery bypass grafting; MI: myocardial infarction; PCI: percutaneous coronary intervention